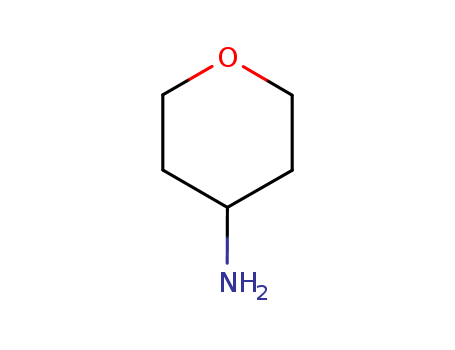
4-Aminotetrahydropyran
4-Aminotetrahydropyran
Purity 99% Min 4-Aminotetrahydropyran 38041-19-9 Spot Supply with Safe Transportation
- Molecular Formula:C5H11NO
- Molecular Weight:101.148
- Vapor Pressure:3.68mmHg at 25°C
- Refractive Index:n20/D 1.463
- Boiling Point:151.4 °C at 760 mmHg
- PKA:9.63±0.20(Predicted)
- Flash Point:49.3 °C
- PSA:35.25000
- Density:0.962 g/cm3
- LogP:0.82440
4-Aminotetrahydropyran(Cas 38041-19-9) Usage
|
Application |
4-Aminotetrahydropyran can be used as reactant/reagent in synthesis of aminothiazole compounds for use in treatment of cancer. |
InChI:InChI=1/C5H11NO/c6-5-1-3-7-4-2-5/h5H,1-4,6H2/p+1
38041-19-9 Relevant articles
Synthesis method for 4-Aaminotetrahydropyran synthesis
-
Paragraph 0021; 0029; 0034; 0034; 0037; 0038; 0039-0041, (2018/06/23)
The invention discloses a synthesis meth...
Preparation method of important intermediate 4-aminotetrahydropyran
-
Paragraph 0019-0025; 0026-0032; 0033-0039; 0040-0053; 0055, (2018/12/14)
The invention discloses a preparation me...
Heterogeneous Catalytic Reductive Amination of Carbonyl Compounds with Ni-Al Alloy in Water as Solvent and Hydrogen Source
Sch?fer, Christian,Ni?anci, Bilal,Bere, Matthew P.,Da?tan, Arif,T?r?k, Béla
, p. 3127 - 3133 (2016/09/09)
The heterogeneous catalytic reductive am...
4-Aminotetrahydropyrans process for one-pot synthesis
-
Paragraph 0014; 0015, (2017/02/02)
The invention discloses a one-kettle met...
38041-19-9 Process route
-
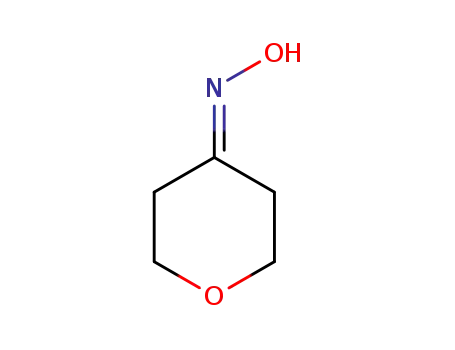
- 61128-73-2
tetrahydropyran-4-one oxime

-
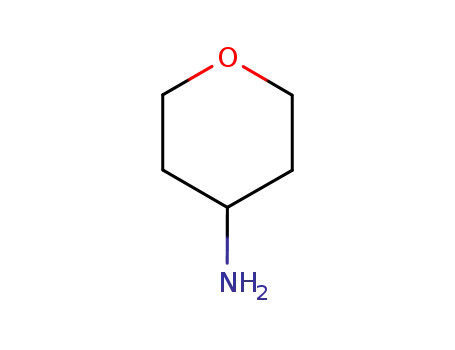
- 38041-19-9
4-aminotetrahydropyran
| Conditions | Yield |
|---|---|
|
With lithium aluminium tetrahydride; In tetrahydrofuran;
|
83% |
|
With hydrogen; nickel; In methanol; at 20 ℃; for 5h;
|
57% |
|
palladium; In ethanol;
|
|
|
palladium; In ethanol; hydrogen;
|
|
|
With hydrogen; palladium; In ethanol;
|
-
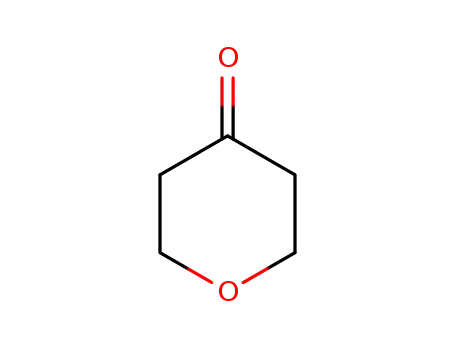
- 29943-42-8,143562-54-3
Tetrahydro-4H-pyran-4-one

-

- 38041-19-9
4-aminotetrahydropyran
| Conditions | Yield |
|---|---|
|
Tetrahydro-4H-pyran-4-one; With ammonium formate; In methanol; water; at 20 ℃;
With palladium on activated charcoal; In methanol; water; at 20 ℃;
|
88% |
|
With ammonium formate; palladium on activated charcoal; In methanol; water; at 20 ℃;
|
80% |
|
Multi-step reaction with 2 steps
1: 100 percent / hydroxylamine hydrochloride; sodium acetate / ethanol / 20 h / Heating
2: 83 percent / LiAlH4 / tetrahydrofuran
With lithium aluminium tetrahydride; hydroxylamine hydrochloride; sodium acetate; In tetrahydrofuran; ethanol;
|
|
|
With ammonium hydroxide; nickel-aluminum alloy; water; at 20 ℃; for 2h; Sonication; Green chemistry;
|
90 %Chromat. |
38041-19-9 Upstream products
-
5337-03-1
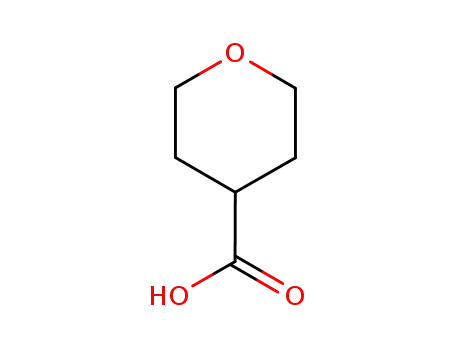
tetrahydro-2H-pyran-4-carboxylic acid
-
344329-76-6
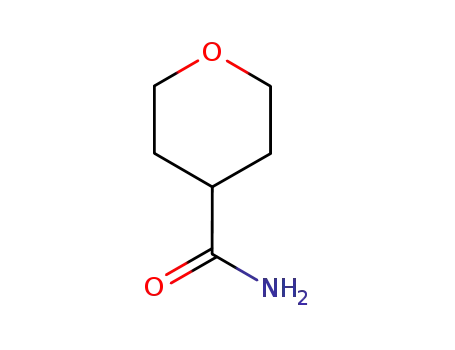
tetrahydro-pyran-4-carboxylic acid amide
-
61128-73-2

tetrahydropyran-4-one oxime
-
60-29-7
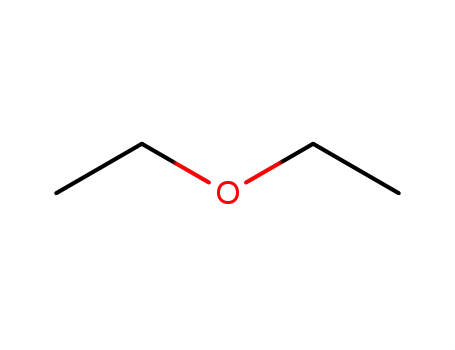
diethyl ether
38041-19-9 Downstream products
-
38035-10-8
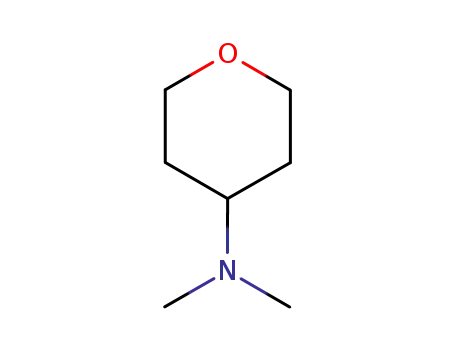
N,N-dimethyl-N-tetrahydro-2H-pyran-4-ylamine
-
91640-86-7
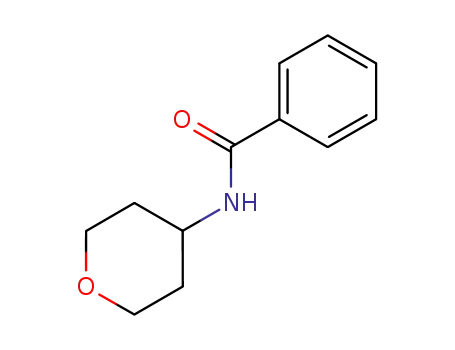
N-(tetrahydro-2H-pyran-4-yl)benzamide
-
959937-52-1
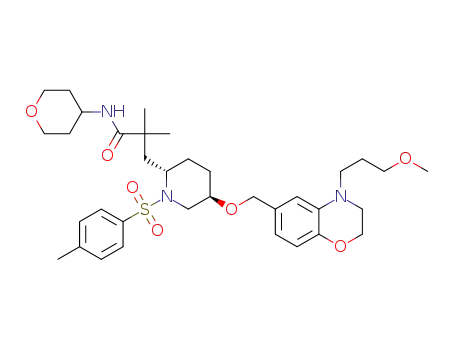
3-[(2S,5R)-5-[4-(3-methoxypropyl)-3,4-dihydro-2H-benzo[1,4]oxazin-6-ylmethoxy]-1-(toluene-4-sulphonyl)piperidin-2-yl]-2,2-dimethyl-N-(tetrahydropyran-4-yl)propionamide
-
1005478-43-2
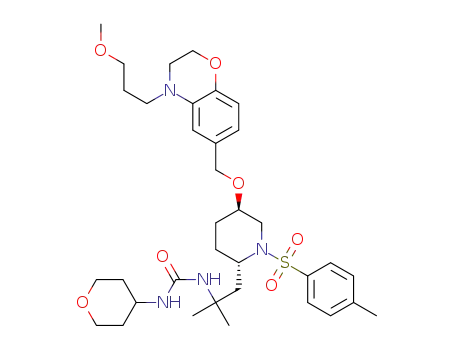
1-{2-[(2S,5R)-5-[4-(3-methoxypropyl)-3,4-dihydro-2H-benzo[1,4]oxazin-6-ylmethoxy]-1-(toluene-4-sulphonyl)piperidin-2-yl]-1,1-dimethylethyl}-3-(tetrahydro-pyran-4-yl)urea








 2254784343
2254784343