5,6-DIHYDRO-2H-PYRAN-2-ONE
5,6-DIHYDRO-2H-PYRAN-2-ONE
Quality Factory Sells Top Purity 99% 5,6-DIHYDRO-2H-PYRAN-2-ONE 3393-45-1 with Safe Delivery
- Molecular Formula:C5H6O2
- Molecular Weight:98.1014
- Vapor Pressure:0.055mmHg at 25°C
- Refractive Index:n20/D 1.483(lit.)
- Boiling Point:233.723 °C at 760 mmHg
- Flash Point:89.106 °C
- PSA:26.30000
- Density:1.12 g/cm3
- LogP:0.48950
5,6-DIHYDRO-2H-PYRAN-2-ONE(Cas 3393-45-1) Usage
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 49, p. 1647, 1984 DOI: 10.1021/jo00183a030Tetrahedron Letters, 25, p. 4783, 1984 DOI: 10.1016/S0040-4039(01)81518-5 |
|
General Description |
Enantioselective conjugate addition of Grignard reagents to 5,6-dihydro-2H-pyran-2-one catalyzed by a chiral phosphine-copper iodide catalyst has been reported. |
InChI:InChI=1/C5H6O2/c6-5-3-1-2-4-7-5/h1,3H,2,4H2
3393-45-1 Relevant articles
Catalytic Allylic Oxidation of Cyclic Enamides and 3,4-Dihydro-2H-Pyrans by TBHP
Yu, Yang,Humeidi, Ranad,Alleyn, James R.,Doyle, Michael P.
, p. 8506 - 8513 (2017/08/23)
Allylic oxidation of heteroatom substitu...
Tandem cross enyne metathesis (CEYM)-intramolecular Diels-Alder reaction (IMDAR). An easy entry to linear bicyclic scaffolds
Miró, Javier,Sánchez-Roselló, María,Sanz, álvaro,Rabasa, Fernando,Del Pozo, Carlos,Fustero, Santos
supporting information, p. 1486 - 1493 (2016/04/09)
A new tandem cross enyne metathesis (CEY...
3393-45-1 Process route
-
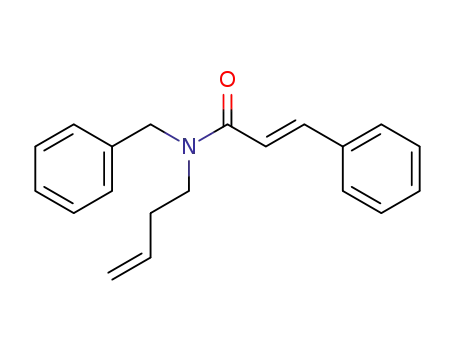
-
(E)-N-benzyl-N-(but-3-en-1-yl)cinnamamide

-
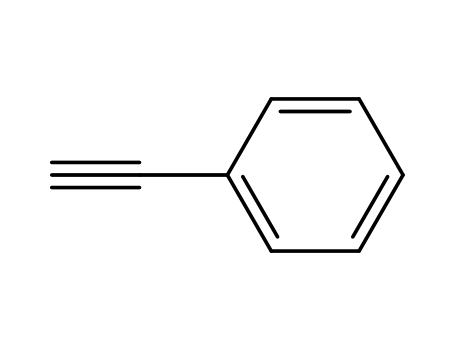
- 536-74-3
phenylacetylene

-
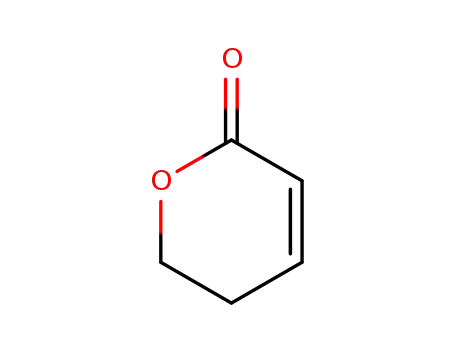
- 3393-45-1
5,6-dihydro-pyran-2-one

-
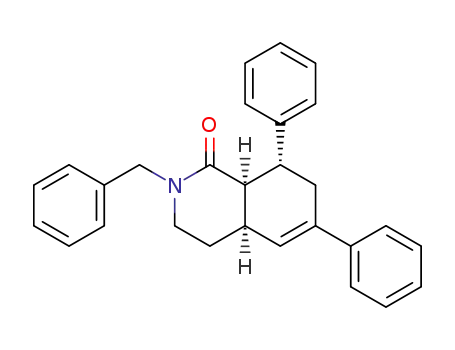
-
(8SR,8aSR)-2-benzyl-6,8-diphenyl-3,4,4a,7,8,8a-hexahydroisoquinolin-1(2H)-one

-
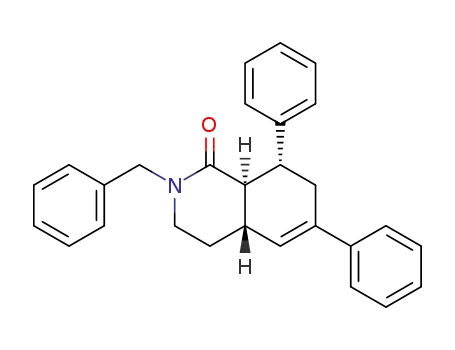
-
(8SR,8aRS)-2-benzyl-6,8-diphenyl-3,4,4a,7,8,8a-hexahydroisoquinolin-1(2H)-one

-
![N-benzyl-N-[(Z)-5-phenylhexa-3,5-dien-1-yl]cinnamamide](/upload/2024/8/bd6e65af-8600-4136-a611-d8c7b0ae3b64.png)
-
N-benzyl-N-[(Z)-5-phenylhexa-3,5-dien-1-yl]cinnamamide
| Conditions | Yield |
|---|---|
|
With [1,3-bis-(2,4,6-trimethylphenyl)-2-imidazolidinylidene]dichloro(O-isopropoxyphenylmethylene)ruthenium; In toluene; at 90 ℃; for 48h; Overall yield = 78 %; diastereoselective reaction; Inert atmosphere; Sealed tube;
|
6 % de |
-
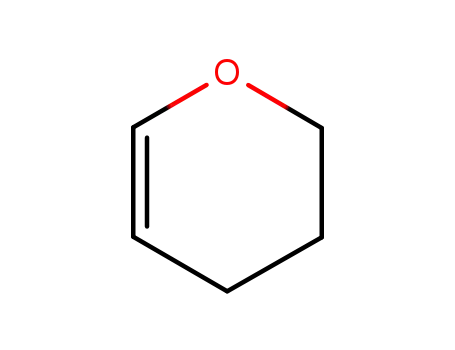
- 110-87-2
3,4-dihydro-2H-pyran

-

- 3393-45-1
5,6-dihydro-pyran-2-one

-
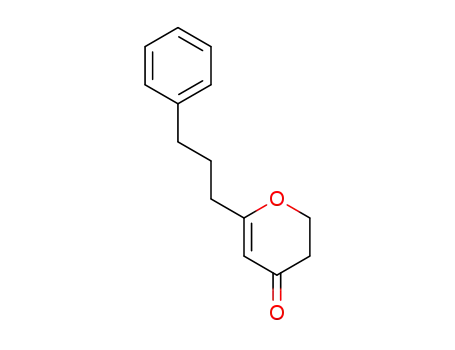
-
6-(3-phenylpropyl)-2H-pyran-4(3H)-one
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1.1: tert.-butyl lithium / tetrahydrofuran; pentane / 1 h / -78 - 0 °C / Inert atmosphere
1.2: 2 h / -78 °C / Inert atmosphere
2.1: tert.-butylhydroperoxide; sodium acetate; dirhodium tetrakis(caprolactamate); oxygen / water; dichloromethane / 20 °C
With tert.-butylhydroperoxide; dirhodium tetrakis(caprolactamate); tert.-butyl lithium; oxygen; sodium acetate; In tetrahydrofuran; dichloromethane; water; pentane;
|
3393-45-1 Upstream products
-
536-74-3

phenylacetylene
-
110-87-2

3,4-dihydro-2H-pyran
3393-45-1 Downstream products
-
183504-77-0
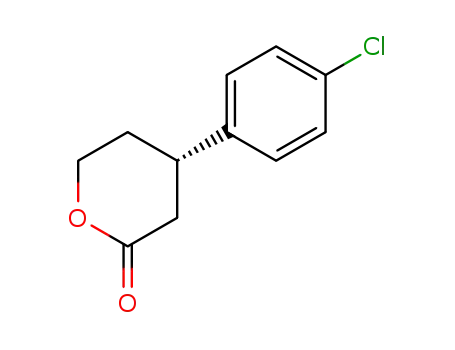
4-(4-chlorophenyl)tetrahydro-2H-pyran-2-one
-
61198-49-0
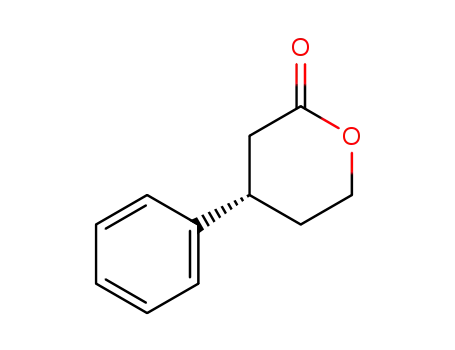
(S)-4-phenyltetrahydro-2H-pyran-2-one
-
156496-88-7
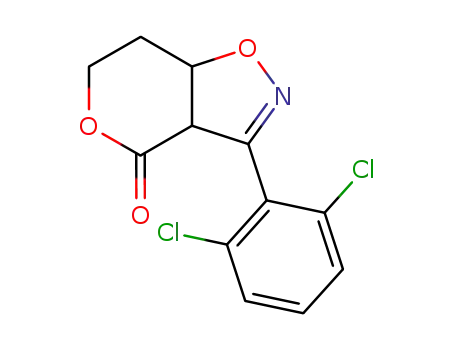
cis-3-(2,6-Dichlorophenyl)-3a,6,7,7a-tetrahydro-4H-pyrano[3,4-d]isoxazol-4-one
-
61898-55-3
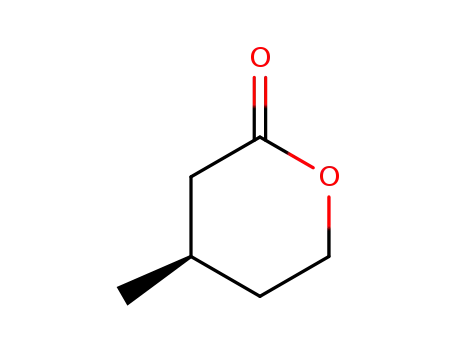
(4R)-4-methyltetrahydro-2H-pyran-2-one
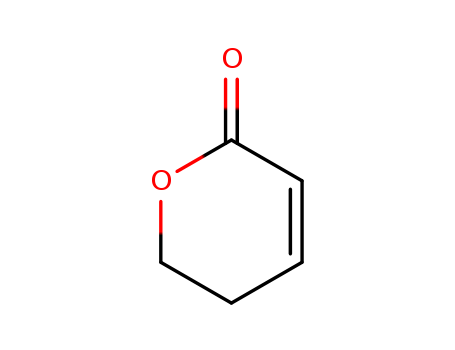








 2254784343
2254784343