Methylisothiazolinone(MIT)
Methylisothiazolinone(MIT)
Quality Factory Sells Top Purity 99% Methylisothiazolinone(MIT) 2682-20-4 with Safe Delivery
- Molecular Formula:C4H5NOS
- Molecular Weight:115.156
- Appearance/Colour:White to yellow powder
- Vapor Pressure:<0.1 mm Hg ( 25 °C)
- Melting Point:254-256 °C(lit.)
- Boiling Point:182.8 °C at 760 mmHg
- PKA:-2.03±0.20(Predicted)
- Flash Point:64.3 °C
- PSA:50.24000
- Density:1.293 g/cm3
- LogP:0.44680
2-Methyl-4-Isothiazolin-3-one(Cas 2682-20-4) Usage
|
Safety |
Methylisothiazolinone (MIT) is a heterocyclic organic compound used as a preservative in cosmetics and personal care products in concentrations up to 0.01%. MIT is a colorless, clear liquid with a mild odor that is completely soluble in water; mostly soluble in acetonitrile, methanol, and hexane; and slightly soluble in xylene. Consistent with its solubility, dermal penetration is low. The Cosmetic Ingredient Review Expert Panel noted the in vitro evidence of neurotoxicity but concluded that the absence of any neurotoxicity findings in the many in vivo studies, including subchronic, chronic, and reproductive and developmental animal studies, suggests that MIT would not be neurotoxic as used in cosmetics. Although recognizing that MIT was a sensitizer in both animal and human studies, the panel concluded that there is a threshold dose response and that cosmetic products formulated to contain concentrations of MIT at 100 ppm (0.0 1%) or less would not be expected to pose a sensitization risk. Accordingly, MIT may be safely used as a preservative in cosmetics up to that concentration. |
|
Definition |
ChEBI: Methylisothiazolinone is a 1,2-thazole that is 4-isothiazolin-3-one bearing a methyl group on the nitrogen atom. It is a powerful biocide and preservative and is the minor active ingredient in the commercial product Kathon(TM). It has a role as an antifouling biocide, an antimicrobial agent and an antifungal agent. |
|
Contact allergens |
MI is generally associated with MCI, in Kathon? CG, MCI/MI, and Euxyl? K 100. This preservative is currently used in water-based products such as cosmetics, paints, and glues. Skin contact with concentrated solution can cause severe irritant dermatitis. |
InChI:InChI=1/C4H5NOS/c1-5-4(6)2-3-7-5/h2-3H,1H3
2682-20-4 Relevant articles
-
Chan,A.W.K. et al.
, p. 2497 - 2506 (1970)
-
Preparation method of 3-isothiazolinone compound
-
Paragraph 0030-0032; 0036-0039, (2020/12/31)
The invention discloses a preparation me...
Preparation method of 2-methyl-4-isothiazoline-3-one aqueous solution
-
Paragraph 0031-0044; 0048-0050, (2020/12/31)
The invention provides a preparation met...
Pipeline type continuous production method of 3-isothiazolinone compound
-
Paragraph 0017, (2019/12/02)
The invention discloses a pipeline type ...
Preparation method of high-purity 2-alkyl-4-isothiazoline-3-ketone
-
Paragraph 0037; 0038; 0039; 0040, (2017/07/19)
The invention discloses a preparation me...
2682-20-4 Process route
-
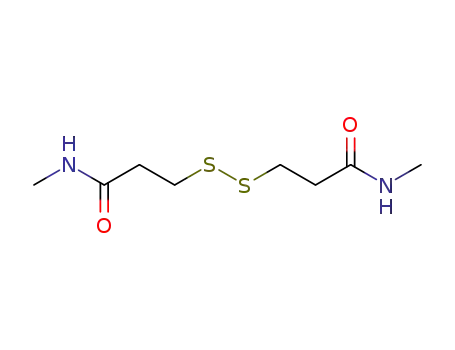
- 999-72-4
N,N'-dimethyl-3,3'-dithiodipropionamide

-
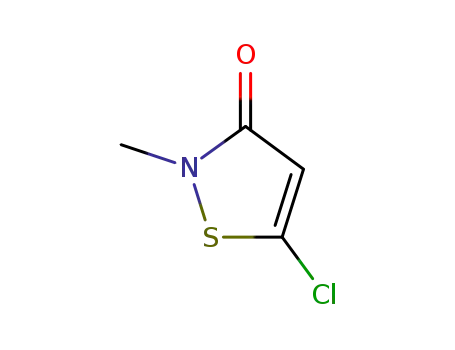
- 26172-55-4
5-chloro-2-methyl-2H-isothiazol-3-one

-
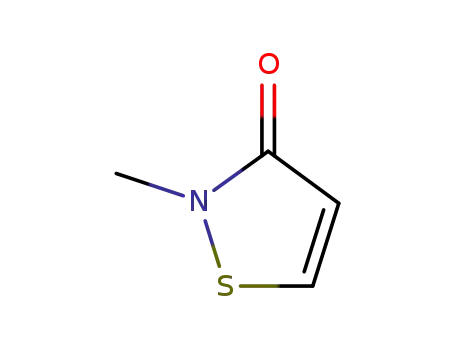
- 2682-20-4
2-methyl-3-isothiazolone
| Conditions | Yield |
|---|---|
|
With thionyl chloride; In 1,2-dichloro-ethane; at 10 ℃; for 2h;
|
56% 18% |
|
With chlorine; In ethyl acetate; acetonitrile; at 5 - 10 ℃; for 1h; Solvent; Temperature; Overall yield = 85 percent;
|
-
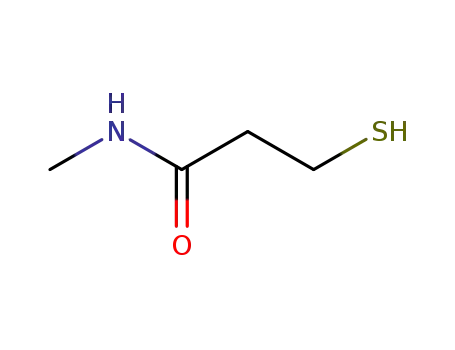
- 52334-99-3
N-methyl-3-mercaptopropionamide

-

- 26172-55-4
5-chloro-2-methyl-2H-isothiazol-3-one

-

- 2682-20-4
2-methyl-3-isothiazolone
| Conditions | Yield |
|---|---|
|
With chlorine; In N,N-dimethyl acetamide; chlorobenzene; at 40 - 45 ℃; for 1h; Overall yield = 74 percent;
|
|
|
N-methyl-3-mercaptopropionamide; With acetyl chloride; In benzene; at 25 ℃; for 0.666667h;
With chlorine; In benzene; at 25 ℃; for 1h; Reagent/catalyst; Temperature; Solvent; Overall yield = 86.2 percent;
|
2682-20-4 Upstream products
-
999-72-4

N,N'-dimethyl-3,3'-dithiodipropionamide
-
77052-39-2
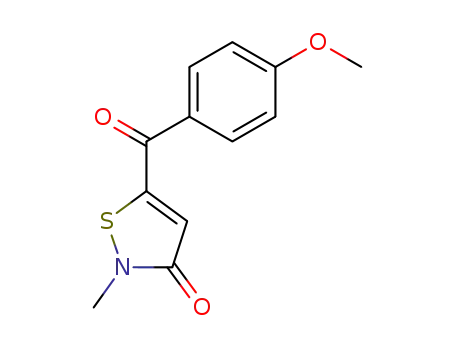
5-(4-Methoxy-benzoyl)-2-methyl-isothiazol-3-one
-
614-45-9
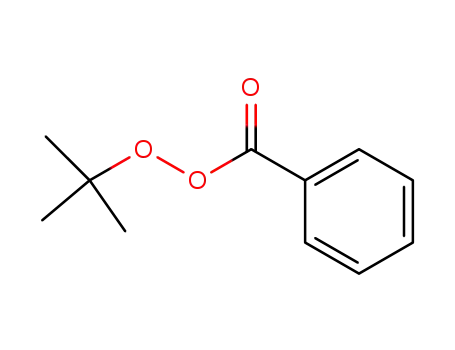
tert-Butyl peroxybenzoate
-
1003-22-1
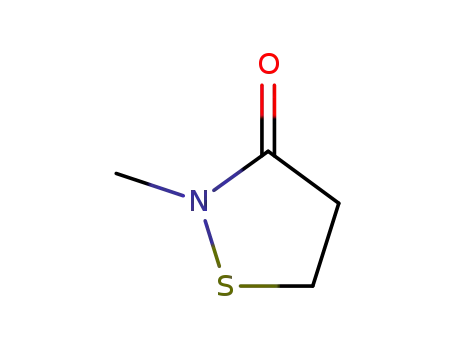
2-methyl-isothiazolidin-3-one
2682-20-4 Downstream products
-
26530-20-1
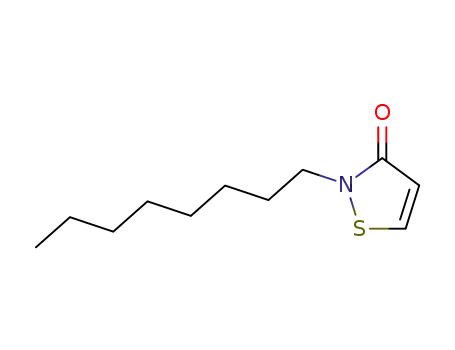
2-octyl-isothiazol-3-one
-
26542-23-4
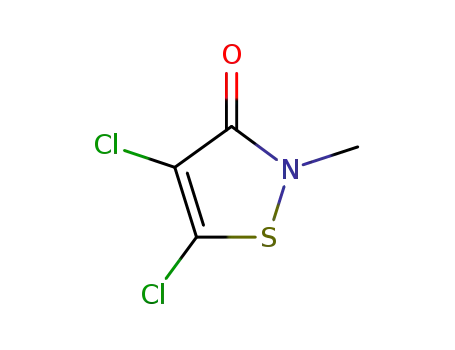
4,5-Dichloro-2-methyl-3(2H)-isothiazolone
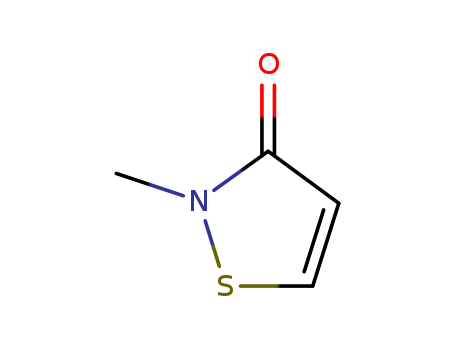








 2254784343
2254784343