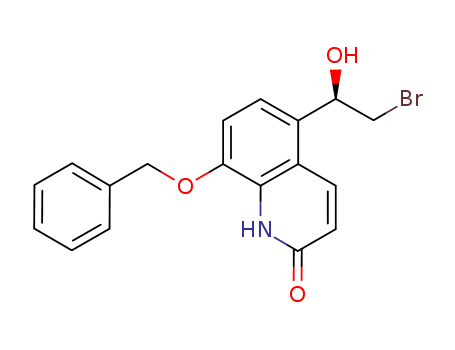
(R)-8-(Benzyloxy)-5-(2-bromo-1-hydroxyethyl)quinolin-2(1H)-one
(R)-8-(Benzyloxy)-5-(2-bromo-1-hydroxyethyl)quinolin-2(1H)-one
Purity 99% Min (R)-8-(Benzyloxy)-5-(2-bromo-1-hydroxyethyl)quinolin-2(1H)-one 530084-79-8 Spot Supply with Safe Transportation
- Molecular Formula:C18H16BrNO3
- Molecular Weight:374.234
- Vapor Pressure:0mmHg at 25°C
- Refractive Index:1.649
- Boiling Point:595.764 °C at 760 mmHg
- PKA:10.78±0.70(Predicted)
- Flash Point:314.107 °C
- PSA:62.32000
- Density:1.491 g/cm3
- LogP:3.53540
8-Benzyloxy-5-((R)-2-broMo-1-hydroxyethyl)-1H-quinolinone(Cas 530084-79-8) Usage
InChI:InChI=1/C18H16BrNO3/c19-10-15(21)13-6-8-16(18-14(13)7-9-17(22)20-18)23-11-12-4-2-1-3-5-12/h1-9,15,21H,10-11H2,(H,20,22)/t15-/m0/s1
530084-79-8 Relevant articles
Discovery of a novel class of inhaled dual pharmacology muscarinic antagonist and β2 agonist (MABA) for the treatment of chronic obstructive pulmonary disease (COPD)
Rancati, Fabio,Linney, Ian D.,Rizzi, Andrea,Delcanale, Maurizio,Knight, Chris K.,Schmidt, Wolfgang,Pastore, Fiorella,Riccardi, Benedetta,Mileo, Valentina,Carnini, Chiara,Cesari, Nicola,Blackaby, Wesley P.,Patacchini, Riccardo,Carzaniga, Laura
supporting information, (2021/04/12)
The targeting of both the muscarinic and...
IMPROVED PROCESS FOR THE PREPARATION OF INDACATEROL MALEATE
-
Page/Page column 9, (2020/10/19)
An improved process for the preparation ...
CLASS OF BIFUNCTIONAL COMPOUNDS WITH QUATERNARY AMMONIUM SALT STRUCTURE
-
Paragraph 0160, (2019/11/11)
The invention provides a class of compou...
8 - Benzyloxy -5 - (R - 2 - bromo -1 - hydroxy-ethyl) - 1H - quinoline -2 - ketone
-
Paragraph 0021; 0022; 0024, (2019/01/08)
The present invention relates to 8 - ben...
530084-79-8 Process route
-

- 100331-89-3
8-benzyloxy-5-(2-bromoacetyl)-1H-quinolin-2-one

-
![8-(benzyloxy)-5-[(1R)-2-bromo-1-hydroxyethyl]quinolin-2(1H)-one](/upload/2024/8/42a03144-71a8-448b-894a-86145a6d3cdf.png)
- 530084-79-8
8-(benzyloxy)-5-[(1R)-2-bromo-1-hydroxyethyl]quinolin-2(1H)-one
| Conditions | Yield |
|---|---|
|
With (S,S)-1,2-diphenyl-1,2-diaminoethane; hydrogen; potassium hydroxide; In isopropyl alcohol; at 25 ℃; under 3800.26 Torr; Solvent; Pressure; Temperature; Autoclave; Inert atmosphere;
|
99% |
|
8-benzyloxy-5-(2-bromoacetyl)-1H-quinolin-2-one; With dimethylsulfide borane complex; (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole; In tetrahydrofuran; at 0 - 30 ℃; Inert atmosphere;
With hydrogenchloride; In tetrahydrofuran; at 0 - 30 ℃;
|
95% |
|
With dimethylsulfide borane complex; (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole; In tetrahydrofuran; at 0 - 10 ℃; Inert atmosphere;
|
95% |
|
With dimethylsulfide borane complex; (1R,2S)-1-Amino-2-indanol; In tetrahydrofuran; at 20 - 25 ℃; for 3.5h; Inert atmosphere;
|
85.5% |
|
With Trimethylboroxine; (R)-α,α-diphenylprolinol; In toluene; at 20 - 150 ℃;
8-benzyloxy-5-(2-bromoacetyl)-1H-quinolin-2-one; With borane; In tetrahydrofuran; toluene; at -10 ℃; for 4.75h;
With methanol; In tetrahydrofuran; toluene; at -10 ℃;
|
81% |
|
With borane; (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole; In tetrahydrofuran; toluene; at -10 ℃; for 4.75h;
|
81% |
|
(R)-α,α-diphenylprolinol; With Trimethylboroxine; In toluene; at 20 - 150 ℃; for 4.5h;
8-benzyloxy-5-(2-bromoacetyl)-1H-quinolin-2-one; With borane; In tetrahydrofuran; toluene; at -10 ℃; for 4.75h;
|
81% |
|
8-benzyloxy-5-(2-bromoacetyl)-1H-quinolin-2-one; With borane-THF; In toluene; at -10 ℃; for 4.75h; Cooling with isopropanol-ice;
With methanol; In toluene;
|
81% |
|
With Trimethylboroxine; (R)-α,α-diphenylprolinol; In toluene; at 20 - 150 ℃; for 4.5h;
8-benzyloxy-5-(2-bromoacetyl)-1H-quinolin-2-one; With trimethylborane; In tetrahydrofuran; toluene; at -15 - -5 ℃; for 4.75h; Cooling with ice-isopropanol;
|
81% |
|
With borane; (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole; In tetrahydrofuran; at -10 - 5 ℃; for 4.75h;
|
81% |
|
Trimethylboroxine; (R)-α,α-diphenylprolinol; In toluene; at 20 - 150 ℃; for 4.5h;
8-benzyloxy-5-(2-bromoacetyl)-1H-quinolin-2-one; With borane; In tetrahydrofuran; methanol; toluene; at -15 - -5 ℃; for 4.75h; Cooling with ice/isopropanol bath;
|
81% |
|
8-benzyloxy-5-(2-bromoacetyl)-1H-quinolin-2-one; With Trimethylboroxine; borane; (R)-α,α-diphenylprolinol; In tetrahydrofuran; toluene; acetonitrile; at -15 - 20 ℃; for 5.25h;
With methanol; In tetrahydrofuran;
|
81% |
|
8-benzyloxy-5-(2-bromoacetyl)-1H-quinolin-2-one; With dimethylsulfide borane complex; (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole; In tetrahydrofuran; toluene; at -20 ℃; for 4h; Inert atmosphere;
With methanol; In tetrahydrofuran; toluene; at -20 - 20 ℃; for 0.333333h;
With hydrogenchloride; In water; at 20 ℃; for 18h;
|
76% |
|
8-benzyloxy-5-(2-bromoacetyl)-1H-quinolin-2-one; With dimethylsulfide borane complex; In tetrahydrofuran; toluene; at -20 ℃; for 3h; Inert atmosphere;
With methanol; In tetrahydrofuran; toluene; at 20 ℃; for 0.333333h;
With hydrogenchloride; In water; at 20 ℃; for 18h;
|
76% |
|
8-benzyloxy-5-(2-bromoacetyl)-1H-quinolin-2-one; With (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole; In tetrahydrofuran; at -20 - 20 ℃; for 4.33333h; Inert atmosphere;
With hydrogenchloride; In water; at 20 ℃; for 18h;
|
76% |
|
With dimethylsulfide borane complex; (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole; In tetrahydrofuran; at -20 ℃; for 4h;
|
76% |
|
With dimethylsulfide borane complex; (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole; In tetrahydrofuran; toluene; at -20 - 20 ℃; Inert atmosphere;
|
76% |
|
8-benzyloxy-5-(2-bromoacetyl)-1H-quinolin-2-one; With (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole; In tetrahydrofuran; toluene; at -20 ℃; Inert atmosphere;
With dimethyl sulfide borane; In tetrahydrofuran; toluene; at -20 ℃; for 4h; Inert atmosphere;
|
76% |
|
With borane dimethyl sulfide complex; (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole; In tetrahydrofuran; toluene; at -20 ℃; for 4h; Inert atmosphere;
|
76% |
|
With dimethylsulfide borane complex; (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole; In tetrahydrofuran; toluene; at -20 ℃; for 4h; Inert atmosphere;
|
76% |
|
With dimethylsulfide borane complex; (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole; In tetrahydrofuran; toluene; at -20 ℃; Inert atmosphere;
|
76% |
|
8-benzyloxy-5-(2-bromoacetyl)-1H-quinolin-2-one; With dimethylsulfide borane complex; Trimethylboroxine; (R)-α,α-diphenylprolinol; In tetrahydrofuran; toluene; at -6 - -5 ℃; for 3.5h;
With methanol; In tetrahydrofuran; toluene; at -5 - 0 ℃; for 0.5h;
|
|
|
With borane-THF; Trimethylboroxine; (R)-α,α-diphenylprolinol; In tetrahydrofuran; toluene; at -10 ℃; for 4.75h;
|
95 % ee |
|
With borane; (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole; In tetrahydrofuran; at -5 ℃; for 3.75h;
|
|
|
8-benzyloxy-5-(2-bromoacetyl)-1H-quinolin-2-one; (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole; In tetrahydrofuran; at -55 - -45 ℃; for 0.666667h; Inert atmosphere;
With borane-THF; In tetrahydrofuran; at -45 - 0 ℃; Inert atmosphere;
With methanol; In tetrahydrofuran; at 20 ℃;
|
|
|
With formic acid; C25H29ClN2O3RuS; potassium formate; In chloroform; at 25 ℃; for 24h; enantioselective reaction; Inert atmosphere;
|
94 % ee |
|
8-benzyloxy-5-(2-bromoacetyl)-1H-quinolin-2-one; In tetrahydrofuran; at 40 ℃; for 48h;
With borane-THF; (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole; In tetrahydrofuran; toluene; at -10 - 0 ℃; for 4.5h;
|
|
|
8-benzyloxy-5-(2-bromoacetyl)-1H-quinolin-2-one; With dimethylsulfide borane complex; (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole; In tetrahydrofuran; toluene; at 0 - 2 ℃; for 3.25 - 3.33333h;
With methanol; In tetrahydrofuran; toluene; for 0.25 - 0.333333h;
|
-
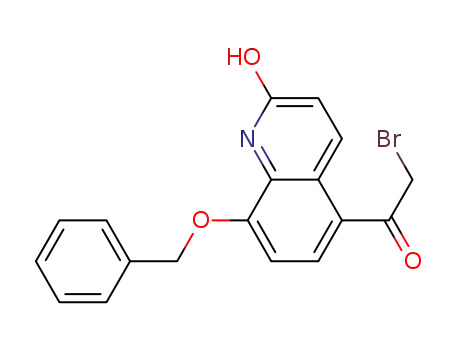
- 100331-89-3
8-benzyloxy-5-bromoacetylcarbostyril

-
![(3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole](/upload/2024/8/262460df-a422-4c64-ad11-f77ba85c414b.png)
- 112022-83-0,133261-83-3
(3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole

-
![8-(benzyloxy)-5-[(1R)-2-bromo-1-hydroxyethyl]quinolin-2(1H)-one](/upload/2024/8/42a03144-71a8-448b-894a-86145a6d3cdf.png)
- 530084-79-8
8-(benzyloxy)-5-[(1R)-2-bromo-1-hydroxyethyl]quinolin-2(1H)-one
| Conditions | Yield |
|---|---|
|
(3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole; With dimethyl sulfide borane; In tetrahydrofuran; at 0 ℃; for 0.166667h;
8-benzyloxy-5-bromoacetylcarbostyril; In tetrahydrofuran; at -10 ℃; for 0.333333h;
With dimethyl sulfide borane; at 0 ℃; for 5h;
|
530084-79-8 Upstream products
-
100331-89-3

8-benzyloxy-5-(2-bromoacetyl)-1H-quinolin-2-one
-
100331-89-3

8-benzyloxy-5-bromoacetylcarbostyril
-
112022-83-0
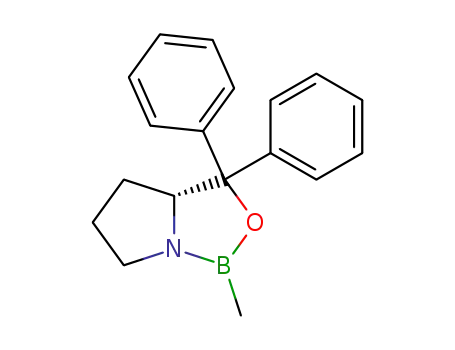
(3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole
-
1127-45-3
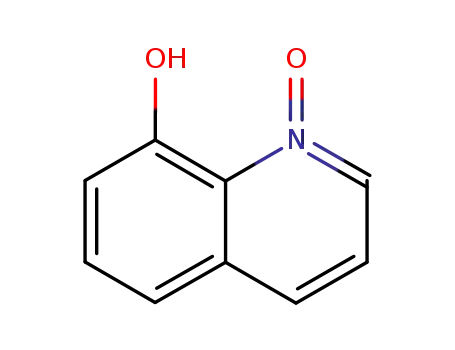
8-Hydroxyquinoline-N-oxide
530084-79-8 Downstream products
-
662111-14-0
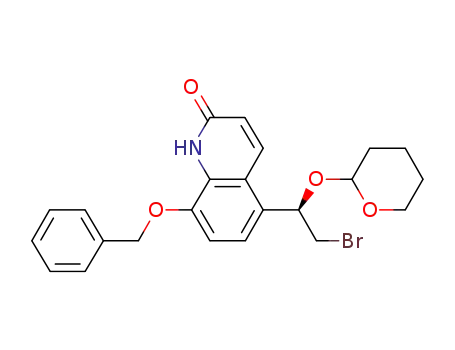
8-(benzyloxy)-5-[(1R)-2-bromo-1-(tetrahydro-2H-pyran-2-yloxy)ethyl]quinolin-2(1H)-one
-
530084-74-3
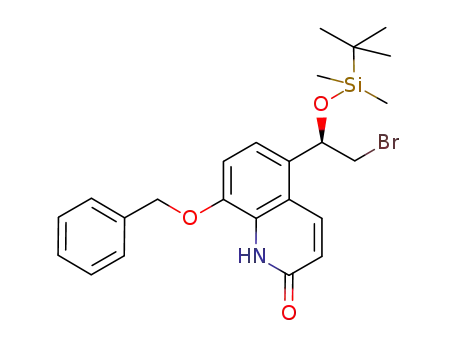
(R)-8-(benzyloxy)-5-(2-bromo-1-((tert-butyldimethylsilyl)oxy)ethyl)quinolin-2(1H)-one
-
173140-90-4
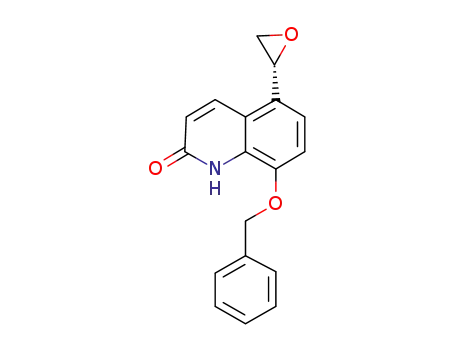
(R)-5-(2-oxiranyl)-8-benzyloxy-2(1H)-quinolinone
-
1254097-58-9
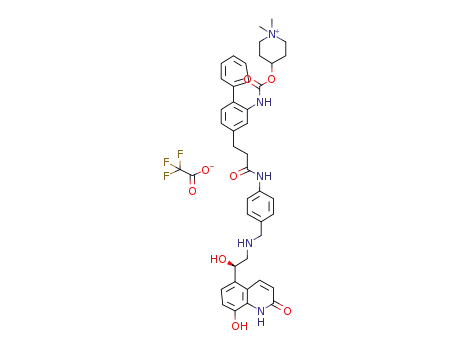
4-({[5-(2-{[4-({[(2R)-2-hydroxy-2-(8-hydroxy-2-oxo-1,2-dihydroquinolin-5-yl)ethyl]amino}methyl)phenyl]carbamoyl}ethyl)-2-phenylphenyl]carbamoyl}oxy)-1,1-dimethylpiperidin-1-ium trifluoroacetate








 2254784343
2254784343