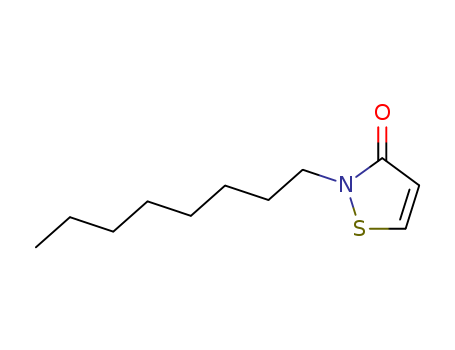
2-Octyl-2H-isothiazol-3-one(OIT)
2-Octyl-2H-isothiazol-3-one(OIT)
Top Quality 2-Octyl-2H-isothiazol-3-one(OIT) 26530-20-1 Hot Sell In Stock
- Molecular Formula:C11H19NOS
- Molecular Weight:213.344
- Appearance/Colour:light yellow oil
- Vapor Pressure:0.00141mmHg at 25°C
- Melting Point:<25 °C
- Refractive Index:1.513
- Boiling Point:304.5 °C at 760 mmHg
- PKA:-2.04±0.20(Predicted)
- Flash Point:137.9 °C
- PSA:50.24000
- Density:1.037 g/cm3
- LogP:3.27030
2-Octyl-2H-isothiazol-3-one(Cas 26530-20-1) Usage
|
Air & Water Reactions |
Insoluble in water. |
|
Reactivity Profile |
2-Octyl-2H-isothiazol-3-one reacts as an isothiocyanate. Isothiocyanates are incompatible with many classes of compounds, reacting exothermically to release toxic gases. Reactions with amines, aldehydes, alcohols, alkali metals, ketones, mercaptans, strong oxidizers, hydrides, phenols, and peroxides can cause vigorous releases of heat. |
|
Fire Hazard |
2-Octyl-2H-isothiazol-3-one is probably combustible. |
|
Flammability and Explosibility |
Nonflammable |
|
Contact allergens |
This isothiazolinone, contained in relatively few products compared to other isothiazolinones, is used in cleaning and polishing agents, latex paints, stains, adhesives, wood and leather preservatives, metalworking fluids (cutting oils), and plastic manufacture. |
|
Safety Profile |
Moderately toxic by ingestion and skin contact. A skin and severe eye irritant. A rmldewcide. When heated to decomposition it emits very toxic fumes of SOx and NOx. See also KETONES. |
|
Potential Exposure |
Isothiazolone/isothiocyanate/heteroaramatic fungicide and microbiocide used on textiles, in metalworking fluids, and some water thinned paints. Its use as a fungicide on cotton was canceled in the United States and the tolerances were revoked in 1998. |
|
Shipping |
UN2922 Corrosive liquids, toxic, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, 6.1-Poisonous materials. |
|
Incompatibilities |
Oxidizers. Contact with hydrogen peroxide may form explosive material. Isothiocyanates are incompatible with many classes of compounds, reacting exothermically to release toxic gases. Reactions with amines, aldehydes, alcohols, alkali metals, ketones, mercaptans, strong oxidizers, hydrides, phenols, and peroxides can cause vigorous releases of heat. Ketones behave a weak acid. Forms water soluble alkali metal salts. Ketones are reactive with many acids and bases liberating heat and flammable gases. The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable hydrogen gas and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. They react violently with aldehydes, nitric acid, and perchloric acid. |
|
Waste Disposal |
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Under 40 CFR 261.5 small quantity generators of this waste may qualify for partial exclusion from hazardous waste regulations. |
|
Definition |
ChEBI: A member of the class of 1,2-thiazole that is 1,2-thiazol-3-one substituted on the nitrogen (position 2) by an octyl group. A fungicide and antibacterial agent, it is used for treatment of canker and other fungal and bacterial diseases in fruit trees. It i no longer approved for use within the European Union. |
|
General Description |
Clear dark amber liquid. Used as a fungicide. |
InChI:InChI=1/C11H19NOS/c1-3-13-8-4-7-12-9-11-6-5-10(2)14-11/h5-6,12H,3-4,7-9H2,1-2H3
26530-20-1 Relevant articles
Method for coproducing OIT and DCOIT
-
Paragraph 0032-0034; 0036-0038; 0040-0042; 0048; 0049, (2021/01/11)
The invention discloses a method for cop...
Pipeline type continuous production method of 3-isothiazolinone compound
-
Paragraph 0020, (2019/12/02)
The invention discloses a pipeline type ...
A 4, 5 - dichloro - N - [...] thiazolinone preparation method
-
Paragraph 0011; 0027-0032, (2019/02/08)
A preparation method of 4,5-dichloro-N-n...
STABLE COMPOSITIONS OF THIABENDAZOLE AND IODINE-CONTAINING FUNGICIDES
-
, (2015/02/25)
The present invention relates to stable ...
26530-20-1 Process route
-
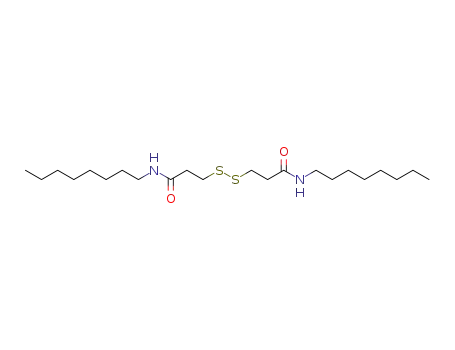
- 33312-01-5
3,3'-disulfanediylbis(N-octylpropanamide)

-
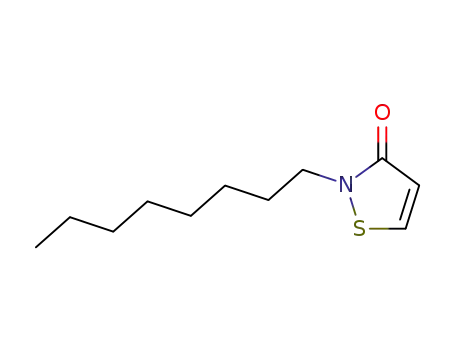
- 26530-20-1
2-octyl-isothiazol-3-one
| Conditions | Yield |
|---|---|
|
With chlorine; sodium iodide; In chlorobenzene; at 30 - 45 ℃; for 0.166667h;
|
88% |
|
With chlorine; In chlorobenzene; at 45 - 55 ℃; Solvent; Temperature; Flow reactor; Large scale;
|
87% |
|
With chlorine; In toluene;
|
|
|
With 2-methoxythiophene; 18-crown-6 ether; titanium tetrachloride; In dichloromethane; at -5 ℃; Reagent/catalyst; Solvent;
|

-

- 26530-20-1
2-octyl-isothiazol-3-one
| Conditions | Yield |
|---|---|
|
|
71% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Hydroxyisothiazol, C8H17X;
|
26530-20-1 Upstream products
-
33312-01-5

3,3'-disulfanediylbis(N-octylpropanamide)
-
111-86-4

n-Octylamine
-
26172-55-4
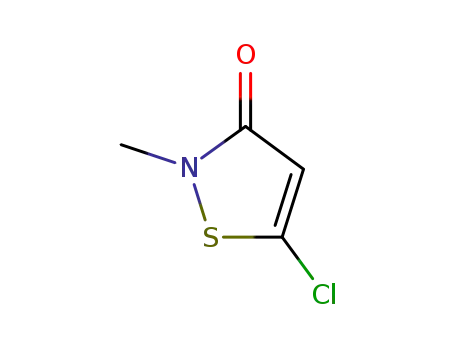
5-chloro-2-methyl-2H-isothiazol-3-one
-
2682-20-4
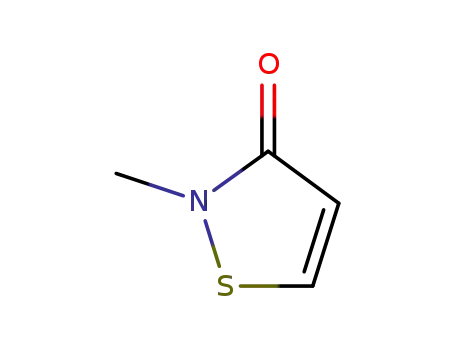
2-methyl-3-isothiazolone
26530-20-1 Downstream products
-
69729-93-7
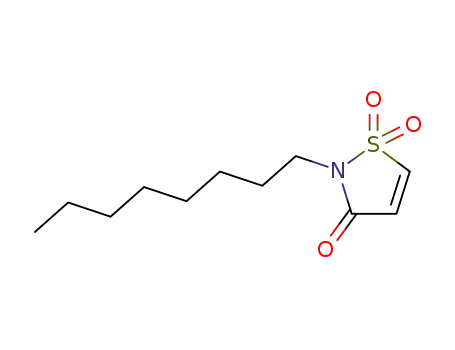
2-octylisothiazol-3(2H)-one 1,1-dioxide
-
137148-53-9
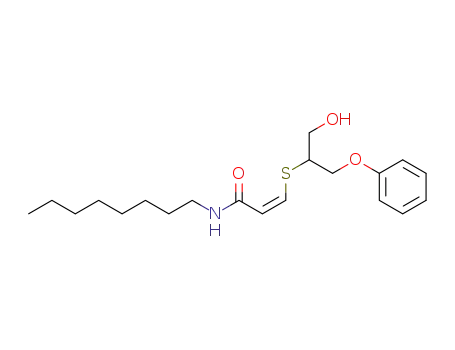
N-octyl-cis-3-[(1-hydroxy-3-phenoxy-2-propane)thio]acrylamide
-
64359-81-5
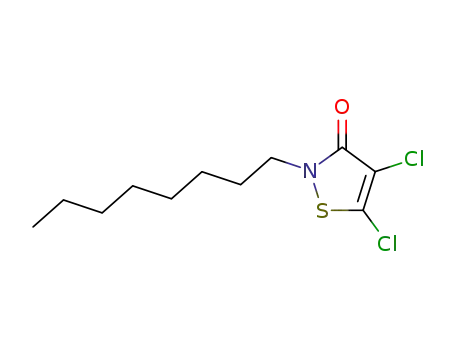
Sea-Nine 211
-
64359-80-4
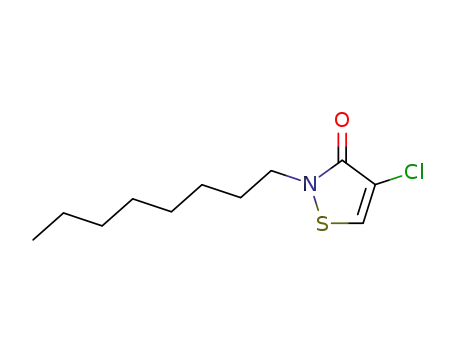
4-chloro-2-octyl-isothiazol-3-one








 2254784343
2254784343