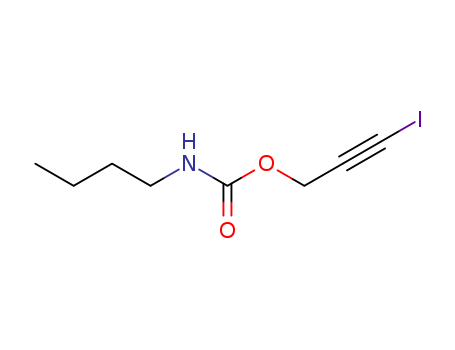
Iodopropynyl butylcarbamate(IPBC)
Iodopropynyl butylcarbamate(IPBC)
Buy Good Quality Iodopropynyl butylcarbamate(IPBC) 55406-53-6 with a minimum purity of 99%
- Molecular Formula:C8H12INO2
- Molecular Weight:281.093
- Appearance/Colour:Off-white solid
- Vapor Pressure:0.005Pa at 25℃
- Melting Point:64-68 °C(lit.)
- Refractive Index:1.546
- Boiling Point:321.8 °C at 760 mmHg
- PKA:12.03±0.46(Predicted)
- Flash Point:148.4 °C
- PSA:38.33000
- Density:1.606 g/cm3
- LogP:2.29950
Iodopropynyl butylcarbamate(Cas 55406-53-6) Usage
|
Application |
1. Cosmetics 2. Wood preservatives 3. Paints 4. Metalworking fluids 5. Household products 6. Moistened toilet tissues 7. Contact lenses 8. Building materials 9. Cooling water 10. Adhesives 11. Textiles 12. Paper |
|
Definition |
ChEBI: A carbamate ester that is carbamic acid in which the nitrogen has been substituted by a butyl group and in which the hydrogen of the carboxy group is replaced by a 1-iodoprop-2-yn-3-yl group. A fungicide, it is used as a preservative and sapstain control c emical in wood products and as a preservative in adhesives, paints, latex paper coating, plastic, water-based inks, metal working fluids, textiles, and numerous consumer products. |
|
Reactivity Profile |
Iodopropynyl butylcarbamate is a carbamate ester. Carbamates are chemically similar to, but more reactive than amides. Like amides they form polymers such as polyurethane resins. Carbamates are incompatible with strong acids and bases, and especially incompatible with strong reducing agents such as hydrides. Flammable gaseous hydrogen is produced by the combination of active metals or nitrides with carbamates. Strongly oxidizing acids, peroxides, and hydroperoxides are incompatible with carbamates. |
|
Flammability and Explosibility |
Notclassified |
|
Contact allergens |
Iodopropynyl butylcarbamate has produced slight irritation in rabbits. However, it was not found to be either a skin sensitizer or a photo sensitizer in guinea pigs. Cosmetic formulations containing up to 0.125% of iodopropynyl butylcarbamate produce no significant irritation or sensitization reactions in human repeated insult patch tests. Iodopropynyl butylcarbamate did not cause crosssensitization reactions in patients who had demonstrated sensitivity to related dithiocarbamate compounds. In the European Union, it is approved as a preservative up to 0.05% and is not to be used in oral hygiene or lip care products. If the concentration exceeds 0.02% in leave-on products, a warning label must indicate that the product contains iodine. |
InChI:InChI=1/C8H12INO2/c1-2-3-6-10-8(11)12-7-4-5-9/h2-3,5-6H2,1H3,(H,10,11)
55406-53-6 Relevant articles
Preparation method for iodopropynyl butylcarbamate
-
Paragraph 0007; 0021-0024, (2018/05/07)
The invention discloses a preparation me...
PROCESS FOR PREPARING IODOPROPARGYL COMPOUNDS
-
Page/Page column 2, (2013/02/28)
Process for the preparation of iodopropa...
PROCESS FOR THE SYNTHESIS OF IODOPROPYNYL BUTYLCARBAMATE IN AN AQUEOUS SOLUTION OF A SUITABLE SURFACTANT
-
Page/Page column 10-11; 12-13, (2008/06/13)
A process for the manufacture of iodopro...
Process for the synthesis of iodopropynyl butylcarbamate in an aqueous solution of a suitable surfactant
-
Page/Page column 3-4, (2008/06/13)
A process for the manufacture of iodopro...
55406-53-6 Process route
-
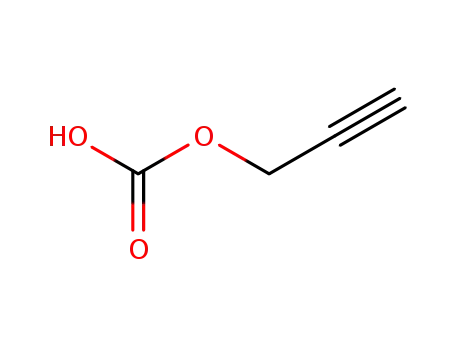
- 57272-05-6
prop-2-yn-1-yl hydrogen carbonate

-
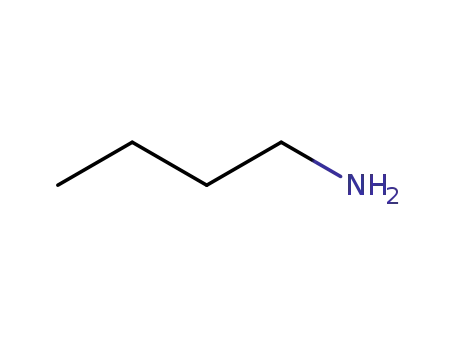
- 109-73-9,85404-21-3
N-butylamine

-
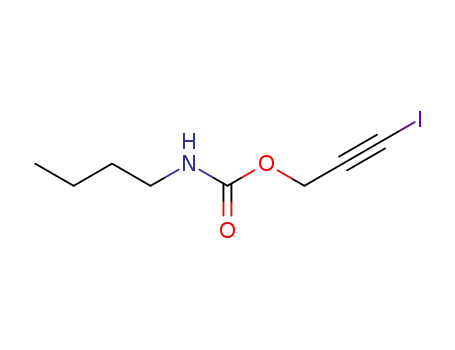
- 55406-53-6
3-iodo-2-propynyl butyl carbamate
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In Dimethyl ether; at 120 ℃; for 12h; Solvent; Industrial scale;
|
91% |
-
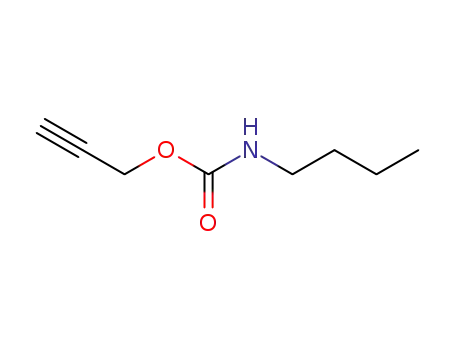
- 76114-73-3
butylcarbamic acid prop-2-ynyl ester

-

- 55406-53-6
3-iodo-2-propynyl butyl carbamate
| Conditions | Yield |
|---|---|
|
butylcarbamic acid prop-2-ynyl ester; With sodium hydroxide; sodium hypochlorite; sodium iodide; In water; at 0 - 40 ℃; for 1.5h;
With acetic acid; In water; at 40 ℃; pH=6.9;
With sodium hydrogensulfite; In water; at 25 - 59 ℃; pH=6.6; Product distribution / selectivity;
|
93.2% |
|
butylcarbamic acid prop-2-ynyl ester; With sodium hydroxide; sodium hypochlorite; potassium iodide; In water; at 0 - 20 ℃; for 1.5h;
With acetic acid; In water; pH=6.9;
With sodium hydrogensulfite; In water; at 55 - 59 ℃; pH=6.6; Product distribution / selectivity;
|
93.5% |
|
butylcarbamic acid prop-2-ynyl ester; With sodium hydroxide; sodium hypochlorite; sodium iodide; In water; at 0 - 20 ℃; for 1.5h;
With acetic acid; In water; pH=6.9;
With sodium hydrogensulfite; In water; at 55 - 59 ℃; pH=6.6; Product distribution / selectivity;
|
93.2% |
|
With chlorine; sodium iodide; sodium hydroxide; In C12-C16-alcohol ethoxylate; Product distribution / selectivity;
|
83% |
|
With sodium hydroxide; sodium chloride; Iodine monochloride; In methanol;
|
|
|
With sodium hydroxide; sodium hypochlorite; iodine; In methanol;
|
55406-53-6 Upstream products
-
76114-73-3

butylcarbamic acid prop-2-ynyl ester
-
57272-05-6

prop-2-yn-1-yl hydrogen carbonate
-
109-73-9

N-butylamine








 2254784343
2254784343