Sodium pyrithione(SPT)
Sodium pyrithione(SPT)
Perfect Factory Offer Excellent quality Sodium pyrithione(SPT) 3811-73-2 with Safe Shipping
- Molecular Formula:C5H4NNaOS
- Molecular Weight:149.149
- Appearance/Colour:clear solution
- Vapor Pressure:0-0Pa at 25℃
- Melting Point:-25 °C
- Refractive Index:1.4825
- Boiling Point:253.8 °C at 760 mmHg
- Flash Point:107.3 °C
- PSA:25.46000
- Density:1.22 g/cm3
- LogP:1.02090
Sodium omadine(Cas 3811-73-2) Usage
|
Biochem/physiol Actions |
2-Mercaptopyridine N-oxide is a zinc ionophore that transports zinc into cells. |
|
Safety Profile |
Poison by intraperitoneal and intravenous routes. Moderately toxic by ingestion, subcutaneous and parenteral routes. Used in preservation of cosmetics. When heated to decomposition it emits very toxic fumes of Na2O, NOx, and SOx. See also MERCAPTANS. |
|
Purification Methods |
When recrystallised from water, it assayed as 98.7% based on AgNO3 titration [Krivis et al. Anal Chem 35 966 1963; see also Krivis et al. Anal Chem 48 1001 1976, and Barton & Crich J Chem Soc, Perkin Trans 1 1603, 1613 1986]. [Beilstein 21/7 V 151.] |
|
Mode of action |
Pyrithione Sodium is the sodium salt form of pyrithione, a fungistatic and antimicrobial derivative of aspergillic acid. Although the exact mechanism of action remains to be fully elucidated, pyrithione sodium appears to interfere with membrane transport ultimately leading to a loss of metabolic control. |
|
Definition |
Apparently exists in equilibrium with the -SH form. Forms chelates with iron, manganese, zinc, etc. |
|
Application |
2-Mercaptopyridine N-oxide sodium salt is one of the active components in paint, sealants, shampoo, adhesive and aerosol due to its anti-microbial activity. In biochemistry studies, it is utilized to transport zinc into cells. Further, it is used to form bidentate oxothiolane chelates with transition metals. It acts as a stabilizer and viscosity building provider in weak basic or neutral medium. |
|
General Description |
2-Mercaptopyridine N-oxide acts as labelling agent during indium-111 labelling of human platelets. It acts as bioactive ligand and forms palladium and platinum complexes, which were tested as potential antitrypanosomal agents. |
InChI:InChI=1/C5H5NOS.Na/c7-6-4-2-1-3-5(6)8;/h1-4,8H;/q;+1
3811-73-2 Relevant articles
Recyclable anhydride catalyst for H2O2 oxidation:: N -oxidation of pyridine derivatives
Gajeles, Ghellyn,Kim, Se Mi,Lee, Kyung-Koo,Lee, Sang Hee,Yoo, Jong-Cheol
, p. 9165 - 9171 (2020/03/13)
The catalytic efficiency and recyclabili...
Novel synthetic method of sodium pyrithione
-
Paragraph 0038; 0040-0041; 0043-0044; 0046-0047; 0049-0050;, (2019/05/08)
The invention discloses a novel syntheti...
A high-quality zinc pyrithione synthetic method (by machine translation)
-
Paragraph 0028; 0031; 0032, (2018/09/11)
The invention discloses a high-quality z...
Synthesis method of zinc pyrithione
-
Paragraph 0043; 0045, (2017/06/02)
The invention discloses a synthesis meth...
3811-73-2 Process route
-
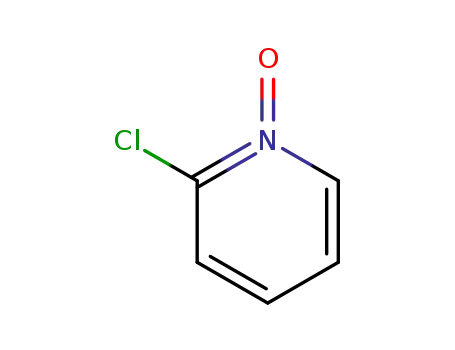
- 2402-95-1
2-chloropyridine-N-oxide

-
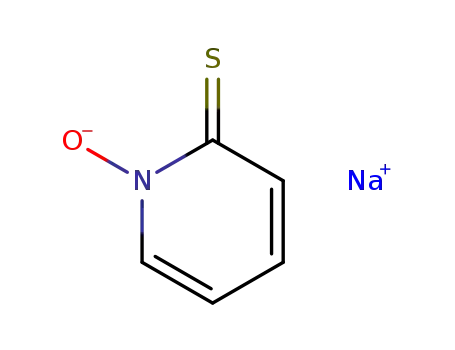
- 3811-73-2
2-mercaptopyridine-1-oxide sodium salt
| Conditions | Yield |
|---|---|
|
With sodium hydrogen sulfide; sodium hydroxide; In water; at 60 - 65 ℃; for 2.5h; Large scale;
|
1218 g |
|
With sodium hydrogen sulfide; sodium hydroxide; at 65 - 80 ℃; for 2h; Green chemistry;
|
544 g |
|
With sodium hydrogen sulfide; sodium hydroxide; at 80 ℃; for 3h;
|
-
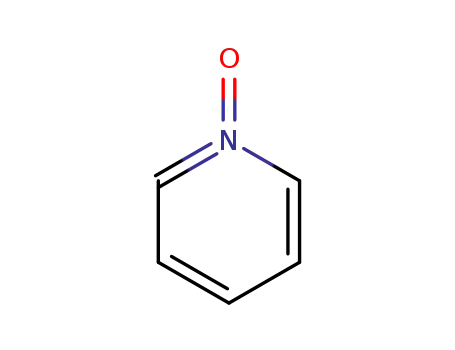
- 694-59-7
pyridine N-oxide

-

- 3811-73-2
2-mercaptopyridine-1-oxide sodium salt
| Conditions | Yield |
|---|---|
|
pyridine N-oxide; With sodium sulfide; 1,3-diisopropylbenzene; sulfur; calcium hydroxide; In water; at 200 ℃; for 3.5h; Inert atmosphere;
With sodium hydroxide; In water; pH=8; Temperature; Time; Reagent/catalyst;
|
|
|
pyridine N-oxide; With sodium sulfide; sodium hexadecyl diphenyl oxide disulfonate; sulfur; calcium hydroxide; at 200 ℃; Autoclave; Inert atmosphere; Reflux;
With hydrogenchloride; In water; pH=3;
With sodium hydroxide; In water; pH=9; Reagent/catalyst; Temperature;
|
3811-73-2 Upstream products
-
694-59-7

pyridine N-oxide
-
109-09-1
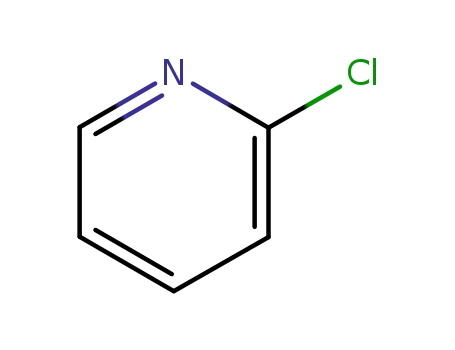
2-chloropyridine
-
2402-95-1

2-chloropyridine-N-oxide
3811-73-2 Downstream products
-
89025-51-4
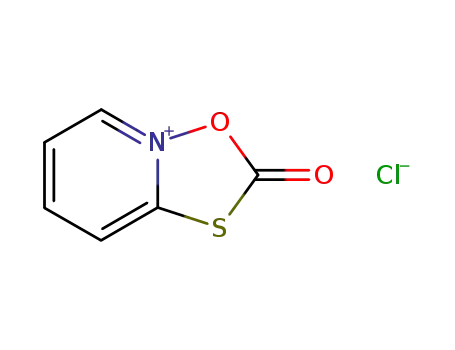
2-oxo-[1,4,2]oxathiazolo[2,3-a]pyridin-4-ium chloride
-
570-74-1
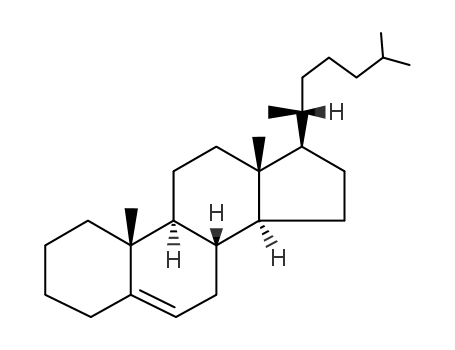
cholest-5-ene
-
4351-55-7
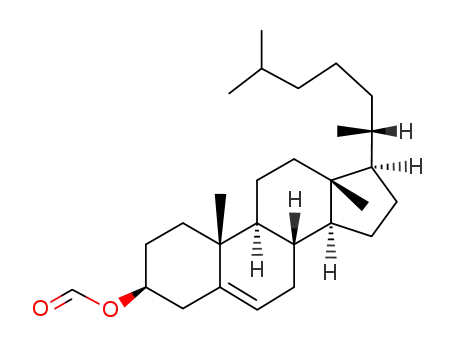
cholesteryl formate
-
93831-98-2
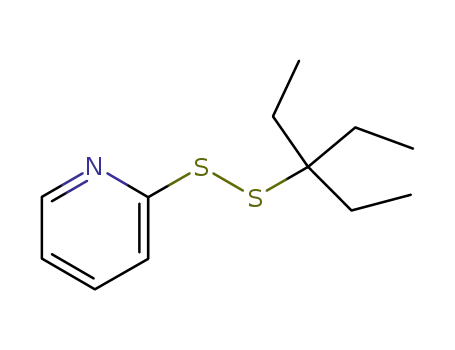
2-pyridyl 1,1-diethylpropyl disulphide








 2254784343
2254784343