(R)-7-(Benzyloxy)-3,4,12,12a-tetrahydro-1H-[1,4]oxazino[3,4-c]pyrido[2,1-f][1,2,4]triazine-6,8-dione
(R)-7-(Benzyloxy)-3,4,12,12a-tetrahydro-1H-[1,4]oxazino[3,4-c]pyrido[2,1-f][1,2,4]triazine-6,8-dione
Chinese Manufacturer Supply (R)-7-(Benzyloxy)-3,4,12,12a-tetrahydro-1H-[1,4]oxazino[3,4-c]pyrido[2,1-f][1,2,4]triazine-6,8-dione 1985607-70-2 On Stock with Competitive Price
- Molecular Formula:C17H17N3O4
- Molecular Weight:327.34
- Boiling Point:575.3±60.0 °C(Predicted)
- Density:1.43±0.1 g/cm3(Predicted)
1985607-70-2 Relevant articles
Synthesis method of baloxavir marboxil intermediate polycyclic carbamoyl pyridone
-
Paragraph 0022-0030, (2021/05/12)
The invention provides a synthesis metho...
A series of new polycyclic carbamoyl pyridone analogues were synthesized by using chloroacetaldehyde as a substrate
Hu, Xueyuan,Kuang, Qiulin,Li, Dan,Wang, Qiang,Wu, Huili,Yuan, Jianyong
supporting information, (2021/06/02)
A facile, universal and economical metho...
Preparation method of fused ring pyridone compound
-
Paragraph 0073-0078; 0079-0082; 0083-0086; 0087-0090, (2021/04/28)
The invention relates to a preparation m...
Preparation method of balosavir intermediate
-
, (2021/01/28)
The invention relates to a preparation m...
1985607-70-2 Process route
-
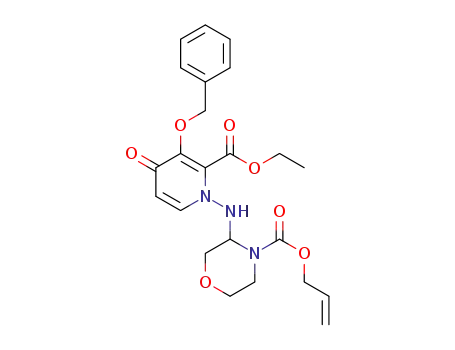
-
allyl 3-((3-(benzyloxy)-2-(ethoxycarbonyl)-4-oxopyridin-1(4H)-yl)amino)morpholine -4-formate

-
![7-(benzyloxy)-3,4,12,12a-tetrahydro-1H-[1,4]oxazino[3,4-c]pyrido [2,1-f][1,2,4]triazine-6,8-diketone](/upload/2024/8/7eedec1f-99ae-4654-8f8f-d34c3c39a2e3.png)
- 1985607-70-2,1370250-39-7
7-(benzyloxy)-3,4,12,12a-tetrahydro-1H-[1,4]oxazino[3,4-c]pyrido [2,1-f][1,2,4]triazine-6,8-diketone
| Conditions | Yield |
|---|---|
|
With morpholine; tetrakis(triphenylphosphine) palladium(0); In tetrahydrofuran; at 20 ℃; for 2h;
|
100% |
|
With morpholine; tetrakis(triphenylphosphine) palladium(0); In tetrahydrofuran; at 20 ℃; for 2h;
|
100% |
|
With morpholine; tetrakis(triphenylphosphine) palladium(0); In tetrahydrofuran; for 3h; Inert atmosphere;
|
90% |
|
With morpholine; tetrakis(triphenylphosphine) palladium(0); In tetrahydrofuran; at 20 ℃; for 2h;
|
|
|
With morpholine; tetrakis(triphenylphosphine) palladium(0); In tetrahydrofuran; at 20 ℃; for 2h;
|
418 mg |
|
With morpholine; tetrakis(triphenylphosphine) palladium(0); In tetrahydrofuran; at 20 ℃; for 2.5h;
|
|
|
With morpholine; tetrakis(triphenylphosphine) palladium(0); In tetrahydrofuran; at 20 ℃; for 2h;
|
418 mg |
-
-
C22H23N3O6

-
![(R)-7-(benzyloxy)-3,4,12,12a-tetrahydro-1H-[1,4]pyrano[4,3-c]pyrido[2,1-f][1,2,4]triazine-6,8(1H,3H)-dione](/upload/2024/8/7420fd12-62d8-4adf-8e29-2e3a9c976a74.png)
- 1985607-70-2,1370250-39-7
(R)-7-(benzyloxy)-3,4,12,12a-tetrahydro-1H-[1,4]pyrano[4,3-c]pyrido[2,1-f][1,2,4]triazine-6,8(1H,3H)-dione
| Conditions | Yield |
|---|---|
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; In ethanol; at 30 ℃; for 0.5h;
|
93.46% |
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; In ethanol; at 20 ℃; for 0.666667h;
|
91% |
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; In ethanol; at 20 ℃; for 0.5h;
|
90% |
|
C22H23N3O6; With 1,8-diazabicyclo[5.4.0]undec-7-ene; In ethanol; at 20 ℃; for 0.5h;
In di-isopropyl ether; at 20 ℃; for 0.5h;
|
89.9% |
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; In ethanol; at 20 ℃; for 0.5h;
|
89.9% |
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; In ethanol; at 20 ℃; for 0.5h;
|
89.9% |
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; In ethanol; at 20 ℃; for 0.5h;
|
89.9% |
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; In methanol; ethyl acetate; at 20 - 30 ℃; for 1h;
|
45% |
1985607-70-2 Upstream products
-
1332855-94-3
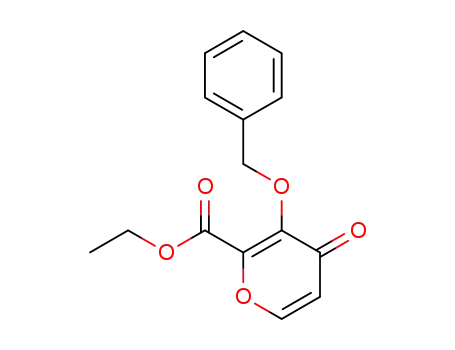
3-(benzyloxy)-4-oxo-4H-pyran-2-carboxylate ethyl ester
-
1985607-66-6
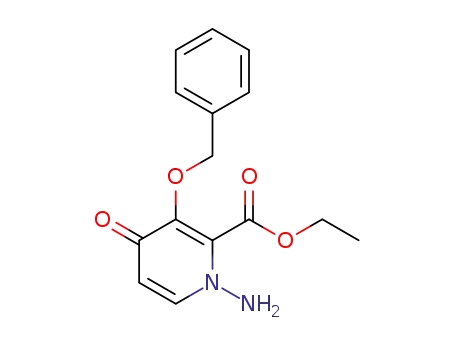
1‐amino‐3‐(benzyloxy)‐4‐oxo-1,4-dihydropyridine‐2‐carboxylic acid ethyl ester
-
119736-16-2
3-(benzyloxy)-1-((tert-butoxycarbonyl)amino)-4-oxo-1,4-dihydropyridine-2-carboxylic acid ethyl ester
-
2136287-59-5
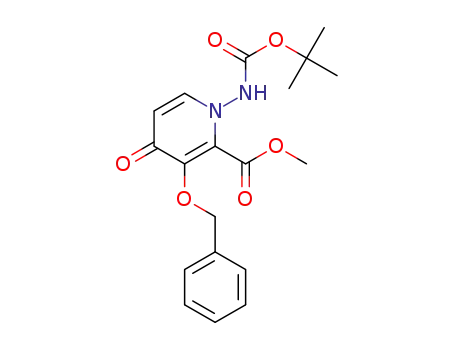
methyl 1-((tert-butoxycarbonyl)amino)-3-(benzyloxy)-4-oxo-1,4-dihydropyridine-2-carboxylic acid
1985607-70-2 Downstream products
-
1985605-59-1
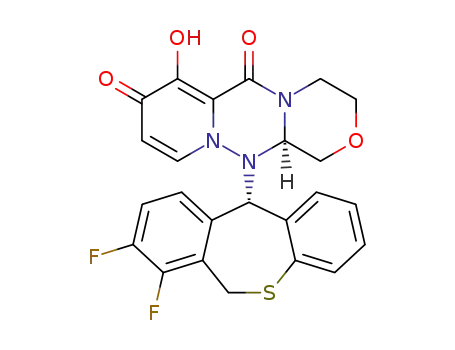
(R)-12-((S)-7,8-difluoro-6,11-dihydrodibenzo[b,e]thiepin-11-yl)-7-hydroxy-3,4,12,12a-tetrahydro-1H-[1,4]oxazino[3,4-c]pyrido[2,1-f][1,2,4]triazine-6,8-dione
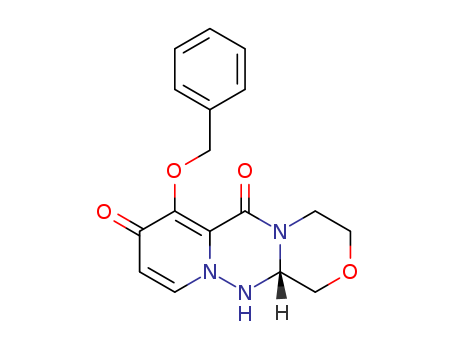








 2254784343
2254784343