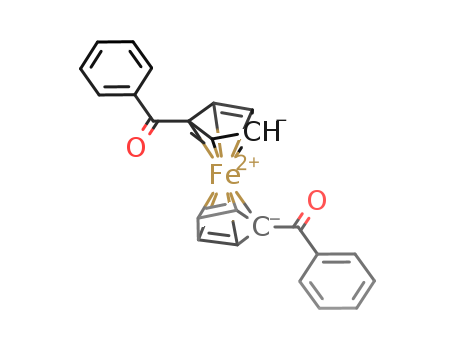
1,1'-Dibenzoylferrocene
1,1'-Dibenzoylferrocene
Buy Good Quality 1,1'-Dibenzoylferrocene 12180-80-2 with a minimum purity of 99%
- Molecular Formula:C24H18FeO2
- Molecular Weight:394.253
- Melting Point:103 °C
- Boiling Point:293.1 °C at 760 mmHg
- Flash Point:124 °C
- PSA:34.14000
- Density:g/cm3
- LogP:4.54670
12180-80-2 Relevant articles
1-Ethyl-3-methylimidazolium halogenoaluminate ionic liquids as solvents for Friedel-Crafts acylation reactions of ferrocene
Stark, Annegret,MacLean, Bonnie L.,Singer, Robert D.
, p. 63 - 66 (1999)
Friedel-Crafts acylations of ferrocene i...
Ruthenium-Catalyzed Enantioselective Hydrogenation of Ferrocenyl Ketones: A Synthetic Method for Chiral Ferrocenyl Alcohols
Lu, Bin,Wang, Qun,Zhao, Mengmeng,Xie, Xiaomin,Zhang, Zhaoguo
, p. 9563 - 9569 (2015/10/12)
Highly effective asymmetric hydrogenatio...
Acceptor-substituted ferrocenium salts as strong, single-electron oxidants: Synthesis, electrochemistry, theoretical investigations, and initial synthetic application
Khobragade, Dushant A.,Mahamulkar, Shraddha G.,Pospí?il, Lubomír,Císa?ová, Ivana,Rulí?ek, Lubomír,Jahn, Ullrich
supporting information, p. 12267 - 12277 (2012/11/14)
A series of mono- and 1,1'-diheteroatom-...
Asymmetric synthesis of planar chiral 2-mono- and 2,2′-disubstituted 1,1′-bisbenzoylferrocenes
Enders, Dieter,Klumpen, Thomas
, p. 698 - 709 (2007/10/03)
An efficient and flexible asymmetric syn...
Efficient regio- and diastereo-controlled synthesis of 1,1′- and 1,1′,2,2′-functionalised ferrocenes and the formation of 2-oxa[3]ferrocenophanes
Carroll, Michael A.,White, Andrew J.P.,Widdowson, David A.,Williams, David J.
, p. 1551 - 1557 (2008/10/08)
The synthesis of a C2 symmetric 1,1′ ,2,...
12180-80-2 Process route
-
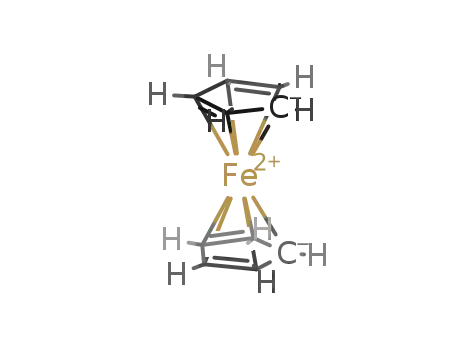
- 102-54-5,55404-68-7
ferrocene

-
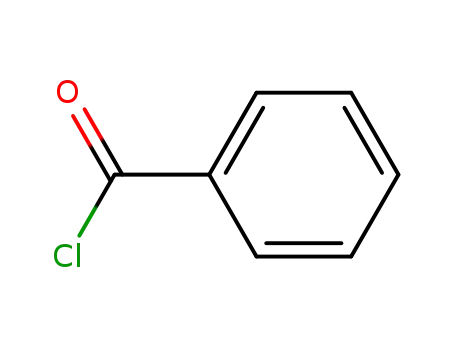
- 98-88-4
benzoyl chloride

-
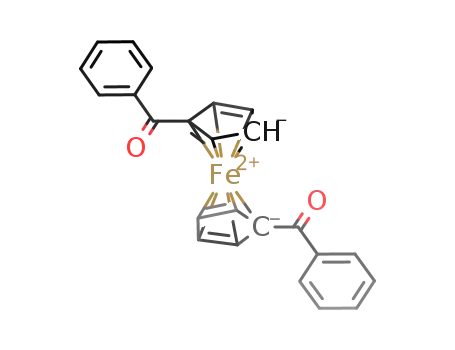
- 12180-80-2,32983-90-7
1,1’-dibenzoylferrocene
| Conditions | Yield |
|---|---|
|
With aluminium chloride; In dichloromethane; ferrocene (1.0 equiv.) added to a stirred suspension of benzoyl chloride(2.2 equiv.) and aluminium chloride (2.2 equiv.) in dichloromethane, st irred at room temp. overnight (16 h); washed with water, organic layer passed through a plug of alumina, washed with chloroform, washings concentrated in vacuo, crude product purified by flash column chromy. on silica gel (TLC Rf 0.26 (1:1 ether-hexane)), elem. anal.;
|
99% |
|
With aluminium trichloride; In dichloromethane; to suspn. of AlCl3 in CH2Cl2 ligand was added, soln. of Fe-complex in CH2Cl2 was added, stirred for 3 ds at room temp. under Ar; aq. soln. of NaHCO3 was added, extd. with CH2Cl2, washed with aq. NaHCO3, dried over MgSO4, concd. under reduced pressure, column chromy. on silica gel with pentane-Et2O; elem. anal.;
|
87% |
|
With aluminium trichloride; benzoyl chloride; In dichloromethane; soln. of C6H5COCl and AlCl3 in dry CH2Cl2 was added dropwise over a period of 1 h to a stirred soln. of ferrocene in dry CH2Cl2; soln. was refluxed for 30 min, hydrolized with 0.1 M HCl, product worked up; solid chromd. on alumina using benzene, following by ether ewluant;
|
86% |
|
With aluminum (III) chloride; In dichloromethane; at 0 ℃; for 0.5h; Inert atmosphere; Schlenk technique;
|
75% |
|
With AlCl3; In dichloromethane;
|
-
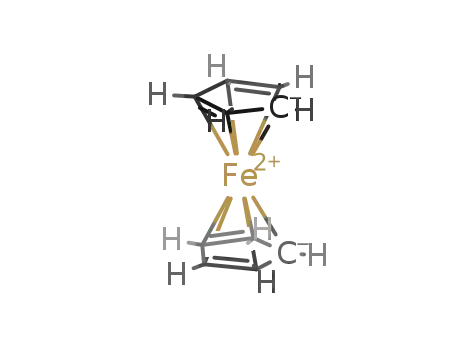
- 102-54-5,55404-68-7
ferrocene

-

- 98-88-4
benzoyl chloride

-
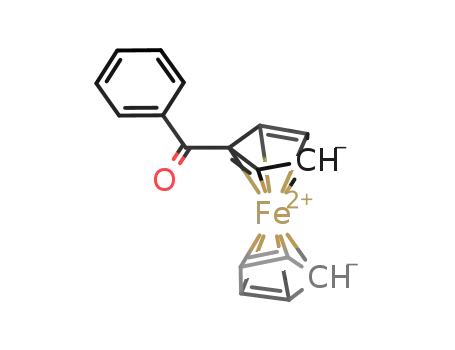
- 1272-44-2
benzoylferrocene

-

- 12180-80-2,32983-90-7
1,1’-dibenzoylferrocene
| Conditions | Yield |
|---|---|
|
With 1-ethyl-3-methylimidazolium iodide-AlCl3; (argon); very slow addn. of AlCl3 to 1-ethyl-3-methylimidazolium iodide, addn. of ferrocene to the ionic liquid, stirring (10 min), ice bath (0°C), addn. of BzCl, stirring (0°C, 2 h), quenching (2 M HCl); extraction (CH2Cl2), drying (MgSO4), concn. (vac.), column chromy. (SiO2, hexanes/ethyl acetate, 10:1 to 1:1);
|
83% 12% |
|
With 1-ethyl-3-methylimidazolium iodide-AlCl3; (argon); very slow addn. of AlCl3 to 1-ethyl-3-methylimidazolium iodide, addn. of ferrocene to the ionic liquid, stirring (10 min), ice bath (0°C), addn. of BzCl, stirring (0°C, 2 h), quenching (2 M HCl); extraction (CH2Cl2), drying (MgSO4), concn. (vac.), column chromy. (SiO2, hexanes/ethyl acetate, 10:1 to 1:1);
|
12% 83% |
|
ferrocene; With n-butyllithium; N,N,N,N,-tetramethylethylenediamine; In hexane; at 20 ℃; Inert atmosphere;
benzoyl chloride; In tetrahydrofuran; dichloromethane; at -78 - 20 ℃; for 2.5h; Inert atmosphere;
|
65% 16% |
12180-80-2 Upstream products
-
102-54-5
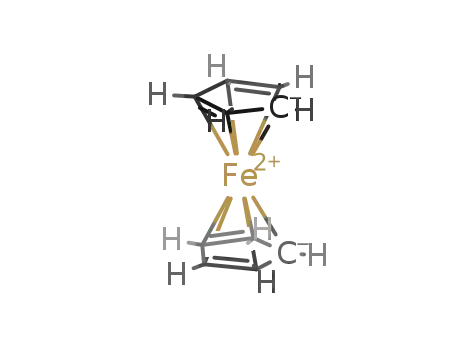
ferrocene
-
85-44-9
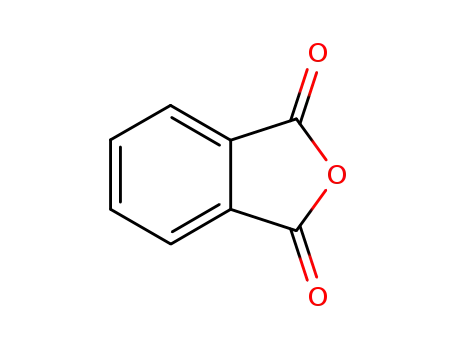
phthalic anhydride
-
98-88-4

benzoyl chloride
12180-80-2 Downstream products
-
251976-64-4
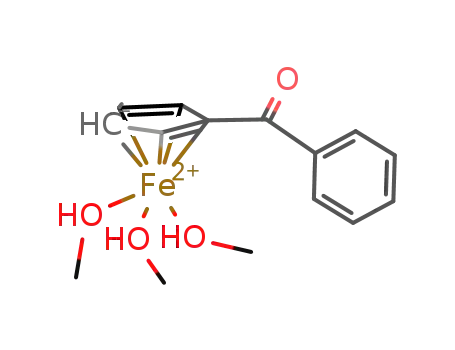
(C5H4COC6H5)Fe(CH3OH)3(1+)
-
12249-22-8
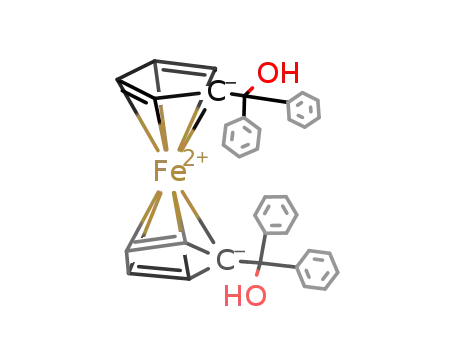
ferrocene-1,1' diylbis(diphenylmethanol)
-
267892-35-3
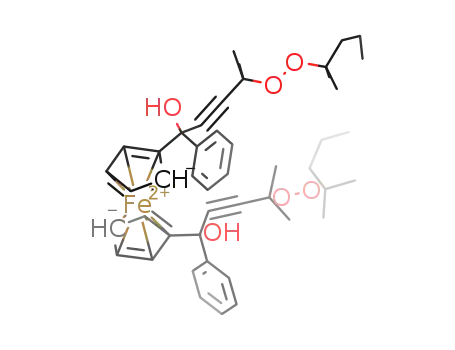
Fe(C5H4C(OH)(C6H5)CCC(CH3)2OOC(CH3)2CH2CH2CH3)2
-
227937-09-9
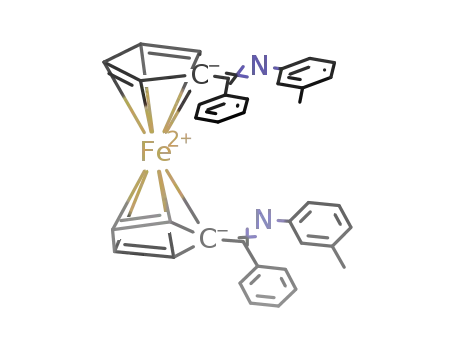
1,1'-bis[(3-methylphenylimino)phenylmethyl]ferrocene








 2254784343
2254784343