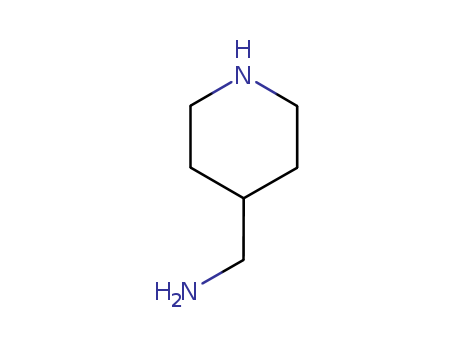
4-(Aminomethyl)piperidine
4-(Aminomethyl)piperidine
Factory Supply industrial standard 4-(Aminomethyl)piperidine 7144-05-0 In Stock
- Molecular Formula:C6H14N2
- Molecular Weight:114.191
- Appearance/Colour:clear colorless to slightly yellow liquid
- Vapor Pressure:0.331mmHg at 25°C
- Melting Point:25 °C(lit.)
- Refractive Index:n20/D 1.49(lit.)
- Boiling Point:200 °C at 760 mmHg
- PKA:10.53±0.10(Predicted)
- Flash Point:78.9 °C
- PSA:38.05000
- Density:0.897 g/cm3
- LogP:0.97380
4-(Aminomethyl)piperidine(Cas 7144-05-0) Usage
|
General Description |
This product has been enhanced for energy efficiency. |
InChI:InChI=1/C6H14N2/c7-5-6-1-3-8-4-2-6/h6,8H,1-5,7H2/p+2
7144-05-0 Relevant articles
Spectrophotometric and thermal studies on the charge - Transfer complexes of 4-(aminomethyl) piperidine as donor with σ- And π-electron acceptors
Mostafa, Adel,El-Ghossein, Nada,Alqaradawi, Siham Y.
, p. 1012 - 1019 (2014)
The spectroscopic characteristics of the...
Corresponding amine nitrile and method of manufacturing thereof
-
Paragraph 0240, (2017/10/22)
The invention relates to a manufacturing...
Electrophilic Zinc Homoenolates: Synthesis of Cyclopropylamines from Cyclopropanols and Amines
Mills, L. Reginald,Barrera Arbelaez, Luis Miguel,Rousseaux, Sophie A. L.
supporting information, p. 11357 - 11360 (2017/08/30)
Metal homoenolates, produced via C-C bon...
Analogs of biologically active, naturally occurring polyamines, pharmaceutical compositions and methods of treatment
-
, (2008/06/13)
Polyamines having the formula: 1or a sa...
7144-05-0 Process route
-
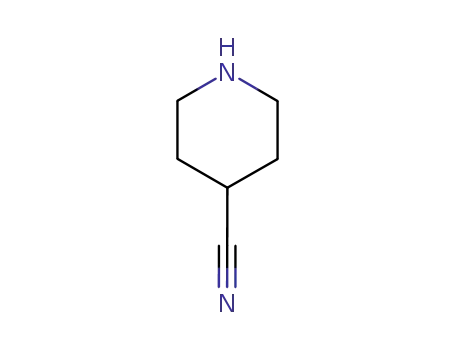
-
4395-98-6
4-cyanopiperidine

-
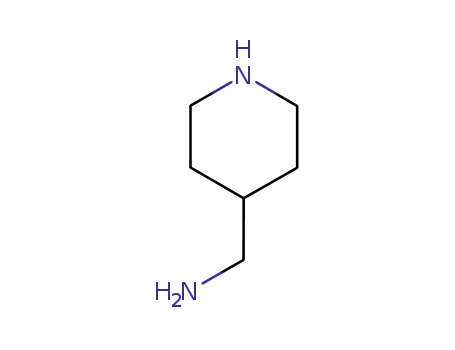
-
7144-05-0
4-Aminomethylpiperidine
| Conditions | Yield |
|---|---|
|
With
hydrogen;
In
ethanol;
at 88 ℃;
for 1h;
under 45004.5 Torr;
|
92% |
|
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
at 0 ℃;
for 16h;
Reflux;
Inert atmosphere;
|
-
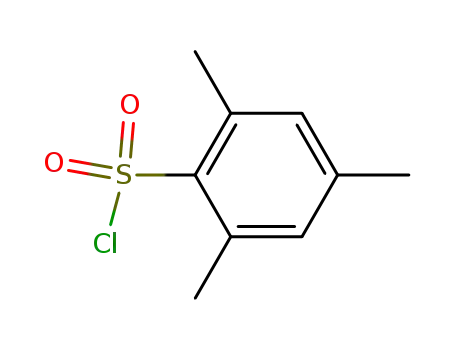
-
773-64-8
2-mesitylenesulphonyl chloride

-
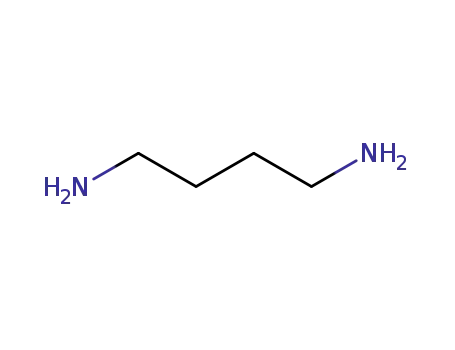
-
110-60-1
1,4-diaminobutane

-

-
7144-05-0
4-Aminomethylpiperidine

-
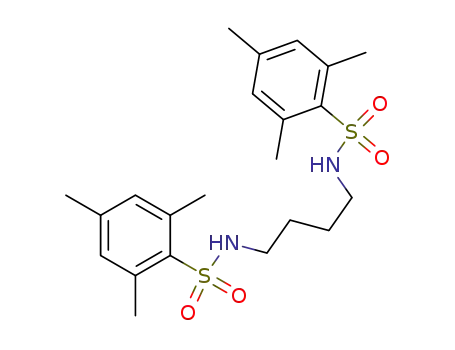
-
161452-28-4
N,N'-bis(2,4,6-trimethylbenzenesulfonyl)-1,4-butanediamine
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
sodium hydroxide; dichloromethane;
|
50.46 g (90%) |
|
With
hydrogenchloride;
In
sodium hydroxide; dichloromethane;
|
50.46 g (90%) |
|
With
hydrogenchloride;
In
sodium hydroxide; dichloromethane;
|
50.46 g (90%) |
7144-05-0 Upstream products
-
132431-14-2
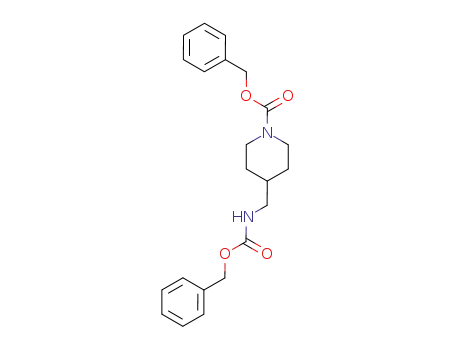
4-(Benzyloxycarbonylamino-methyl)-piperidine-1-carboxylic acid benzyl ester
-
773-64-8

2-mesitylenesulphonyl chloride
-
110-60-1

1,4-diaminobutane
-
4395-98-6

4-cyanopiperidine
7144-05-0 Downstream products
-
144222-22-0
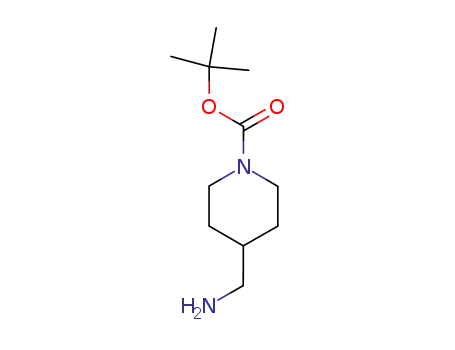
tert-butyl 4-(aminomethyl)piperidine-1-carboxylate
-
65017-57-4
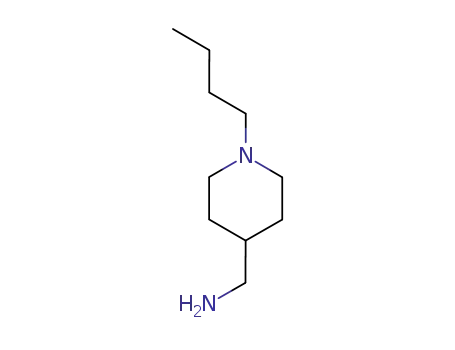
1-(1-butyl-4-piperidinyl)methanamine
-
258345-24-3
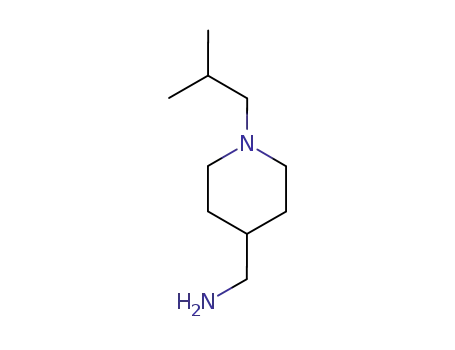
(1-iso-butyl-4-piperidyl)methylamine
-
170353-28-3
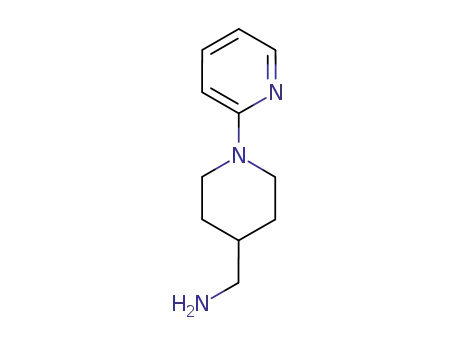
(1-(pyridin-2-yl)piperidin-4-yl)methanamine