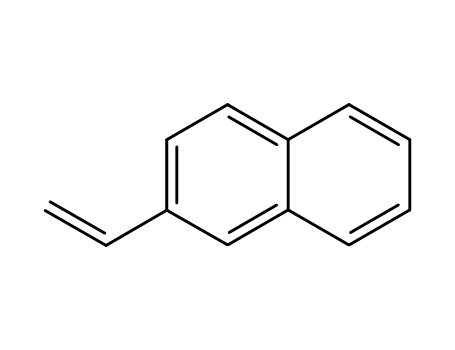
2-Vinylnaphthalene
2-Vinylnaphthalene
Factory sells 2-Vinylnaphthalene 827-54-3 with sufficient production capacity
- Molecular Formula:C12H10
- Molecular Weight:154.211
- Appearance/Colour:Tan powder
- Melting Point:64-68 °C(lit.)
- Boiling Point:270.9 °C at 760 mmHg
- Flash Point:115.5 °C
- PSA:0.00000
- Density:1.031 g/cm3
- LogP:3.48280
2-Vinylnaphthalene(Cas 827-54-3) Usage
|
General Description |
2-Vinylnaphthalene, also known as 2-vinyl-1-naphthalene, is a chemical compound with the formula C12H10. It is a colorless liquid with a sweet, floral odor and is used in the production of plastics and resins. 2-Vinylnaphthalene is a vinylated naphthalene, meaning it has a vinyl group attached to the naphthalene ring. It is used as a precursor in the synthesis of various polymers and as a monomer for the production of vinyl naphthalene resins. It is also used as an intermediate in the manufacturing of pharmaceuticals and fragrances. However, 2-Vinylnaphthalene is also a potential irritant and can cause skin and eye irritation upon contact, as well as harmful effects if inhaled. |
InChI:InChI=1/C12H10/c1-2-10-7-8-11-5-3-4-6-12(11)9-10/h2-9H,1H2
827-54-3 Relevant articles
Photoredox Catalyzed Sulfonylation of Multisubstituted Allenes with Ru(bpy)3Cl2 or Rhodamine B
Chen, Jingyun,Chen, Shufang,Jiang, Jun,Lu, Qianqian,Shi, Liyang,Xu, Zekun,Yimei, Zhao
supporting information, (2021/11/09)
A highly regio- and stereoselective sulf...
Functionalized styrene synthesis via palladium-catalyzed C[sbnd]C cleavage of aryl ketones
Dai, Hui-Xiong,Wang, Xing,Wang, Zhen-Yu,Xu, Hui,Zhang, Xu
supporting information, (2022/03/31)
We report herein the synthesis of functi...
Palladium-Catalyzed Benzylic Silylation of Diarylmethyl Carbonates with Silylboranes under Base-Free Conditions
Asai, Kento,Hirano, Koji,Miura, Masahiro
supporting information, (2022/02/19)
A palladium-catalyzed benzylic silylatio...
Copper-Catalyzed Sulfonylation of Cyclobutanone Oxime Esters with Sulfonyl Hydrazides
Dong, Bingbing,Lu, Jiansha,Bao, Honghao,Zhang, Yuanyuan,Liu, Yingguo,Leng, Yuting
supporting information, p. 3769 - 3776 (2021/07/14)
A copper-catalyzed radical cross-couplin...
827-54-3 Process route
-
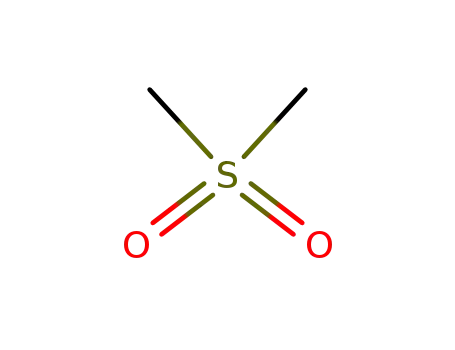
-
67-71-0
dimethylsulfone

-
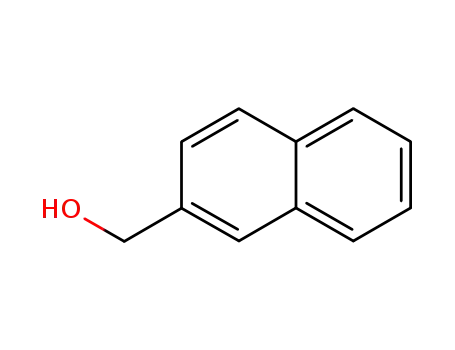
-
1592-38-7
2-Naphthalenemethanol

-
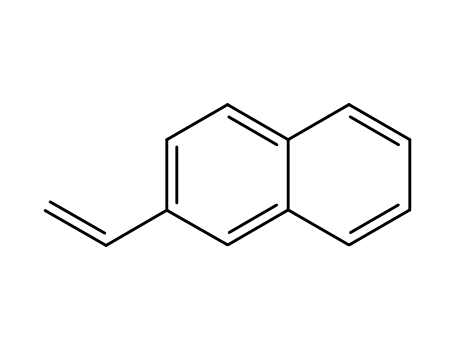
-
827-54-3,28406-56-6
2-naphthylethylene

-
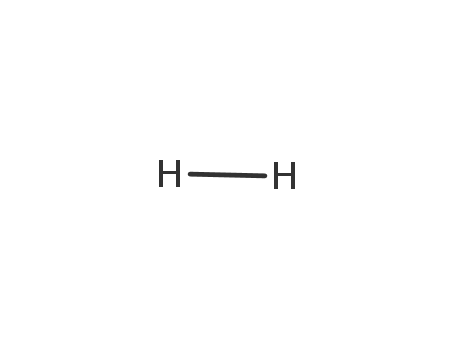
-
1333-74-0
hydrogen
| Conditions | Yield |
|---|---|
|
With
1,10-Phenanthroline; potassium tert-butylate; iron(II) chloride;
In
toluene;
at 120 ℃;
for 24h;
Inert atmosphere;
Schlenk technique;
|
83% |
-
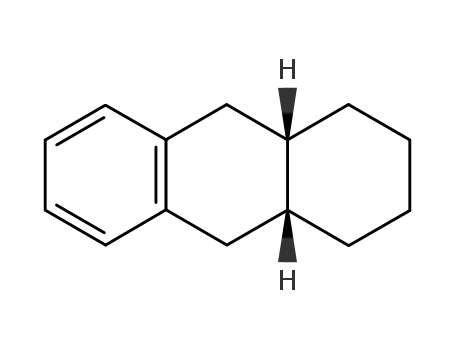
-
64363-88-8
cis-1,2,3,4,4a,9,10,10a-octahydrophenanthrene

-
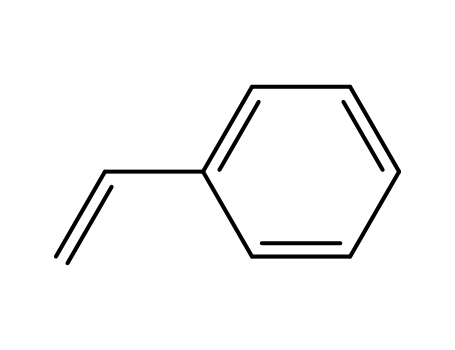
-
100-42-5,25038-60-2,25247-68-1,28213-80-1,28325-75-9,79637-11-9,9003-53-6
styrene

-
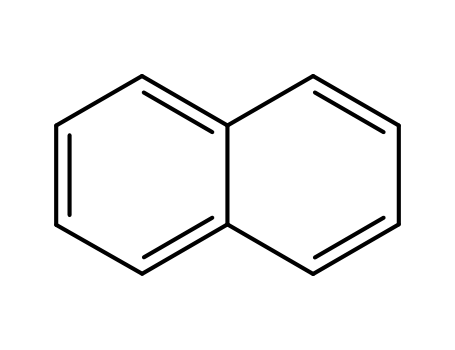
-
91-20-3,71998-51-1,72931-45-4
naphthalene

-
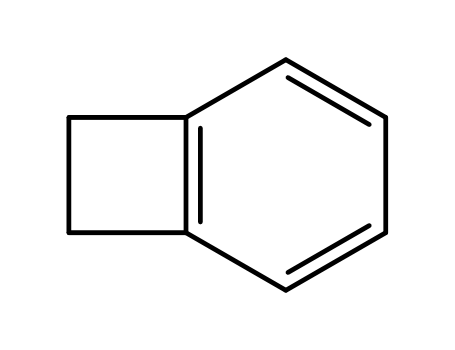
-
694-87-1
benzocyclobutene

-
-
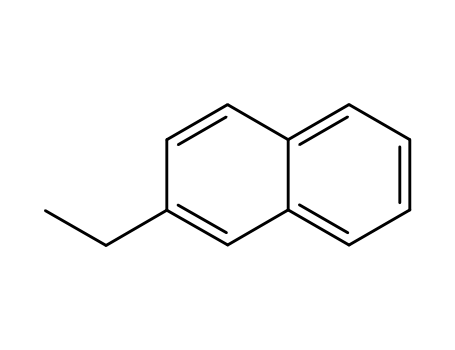
939-27-5
2-ethylnaphthalene

-

-
827-54-3,28406-56-6
2-naphthylethylene

-
-
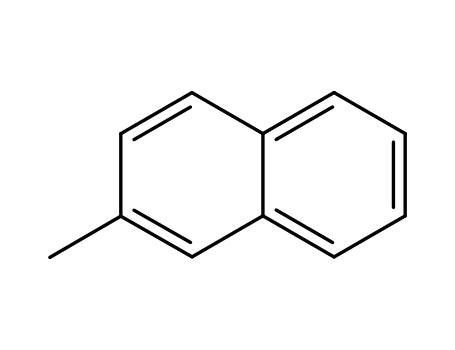
91-57-6,34468-07-0
2-Methylnaphthalene
| Conditions | Yield |
|---|---|
|
at 860 ℃;
under 0.1 Torr;
Product distribution;
other temp.;
|
827-54-3 Upstream products
-
939-27-5

2-ethylnaphthalene
-
1485-07-0
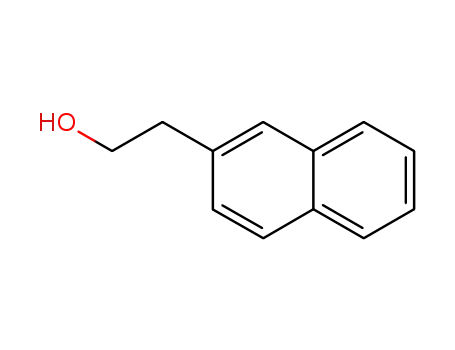
naphthalen-2-ethanol
-
32298-46-7
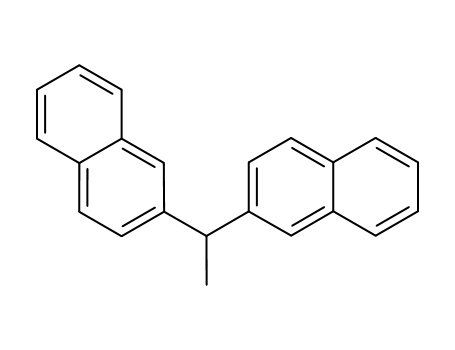
2,2'-(ethane-1,1-diyl)dinaphthalene
-
22364-54-1
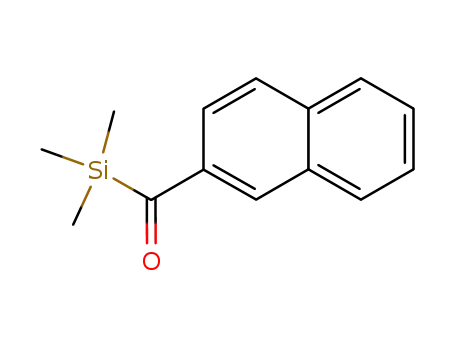
naphthalen-2-yl(trimethylsilyl)methanone
827-54-3 Downstream products
-
143879-48-5
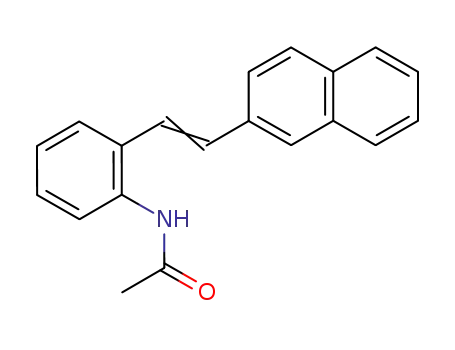
2-<2-(2-naphthyl)vinyl>acetanilide
-
939-27-5

2-ethylnaphthalene
-
136415-67-3
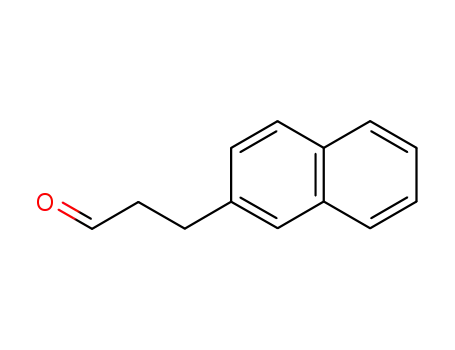
3-(naphthalen-2-yl)propanal
-
159759-69-0
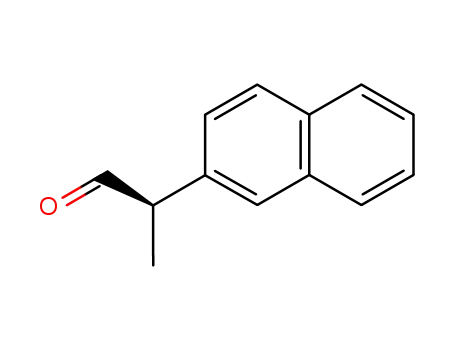
(R)-2-(naphthalen-2-yl)propanal