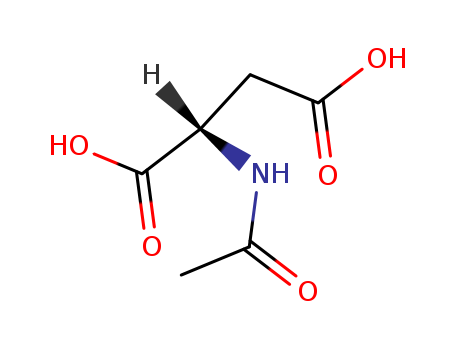
N-acetyl-L-aspartic acid
N-acetyl-L-aspartic acid
Factory supply N-acetyl-L-aspartic acid 997-55-7 with sufficient production capacity
- Molecular Formula:C6H9NO5
- Molecular Weight:175.141
- Appearance/Colour:White powder
- Vapor Pressure:2E-08mmHg at 25°C
- Melting Point:137-140 °C(lit.)
- Boiling Point:425.3 °C at 760 mmHg
- PKA:3.14±0.10(Predicted)
- Flash Point:211 °C
- PSA:103.70000
- Density:1.422 g/cm3
- LogP:-0.55870
N-Acetyl-L-aspartic acid(Cas 997-55-7) Usage
|
Definition |
ChEBI: An N-acyl-L-aspartic acid in which the acyl group is specified as acetyl. |
InChI:InChI=1/C6H9NO5/c1-3(8)7-4(6(11)12)2-5(9)10/h4H,2H2,1H3,(H,7,8)(H,9,10)(H,11,12)/p-2/t4-/m0/s1
997-55-7 Relevant articles
Synthetic strategy of new powerful tris-bisphosphonic ligands for chelation of uranyl, iron, and cobalt cations
Burgada, Ramon,Bailly, Théodorine,Prangé, Thierry,Lecouvey, Marc
, p. 2315 - 2319 (2007)
New tripodal uranyl ion chelators contai...
Peptide Tyrosinase Activators
-
, (2015/06/10)
Peptides that increase melanin synthesis...
Reactions of substituted aspirins with amino acids
Orth, Elisa S.,Medeiros, Michelle,Souza, Bruno S.,Caon, Natalia B.,Kirby, Anthony J.,Nome, Faruk
supporting information, p. 939 - 945 (2014/01/06)
Acyl transfers are key reactions in biol...
Detection of enzyme activity through catalytic signal amplification with functionalized gold nanoparticles
Bonomi, Renato,Cazzolaro, Alessandro,Sansone, Anna,Scrimin, Paolo,Prins, Leonard J.
supporting information; scheme or table, p. 2307 - 2312 (2011/04/21)
A cascade of two catalytic events was us...
Peculiar stability of amino acids and peptides from a radical perspective
Watts, Zachary I.,Easton, Christopher J.
supporting information; experimental part, p. 11323 - 11325 (2011/03/19)
(Chemical Equation Presented) Photochemi...
997-55-7 Process route
-
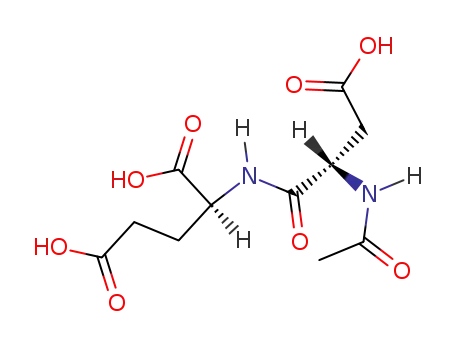
-
3106-85-2
N-acetyl-L-aspartyl-L-glutamate

-
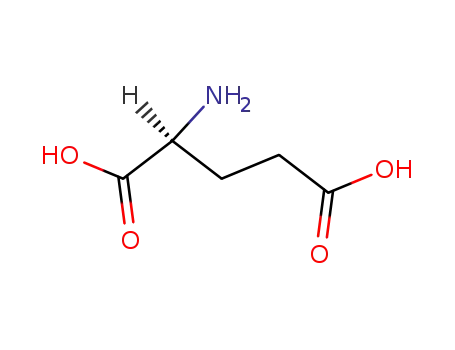
-
56-86-0,21675-62-7,23009-64-5,25104-13-6,84960-48-5,25513-46-6
L-glutamic acid

-
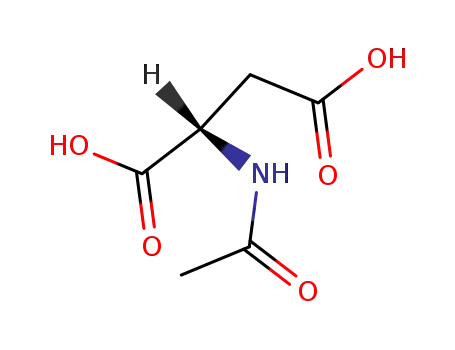
-
997-55-7
N-Acetyl-L-aspartic acid
| Conditions | Yield |
|---|---|
|
With
glutamate carboxypeptidase II; TACN*Zn(II) complex; 2-hydroxypropyl-p-nitrophenyl phosphate;
at 40 ℃;
pH=7;
Kinetics;
aq. buffer;
Enzymatic reaction;
|
-
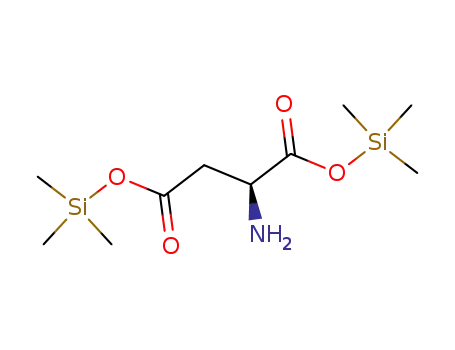
-
5269-42-1
L-Aspartic acid bis(trimethylsilyl) ester

-
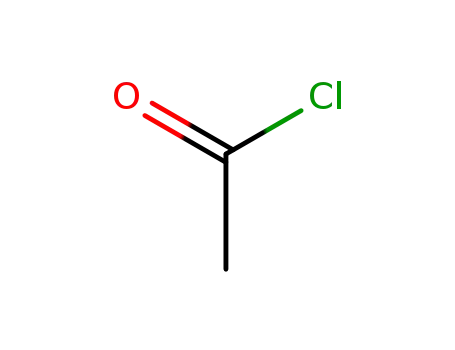
-
75-36-5
acetyl chloride

-

-
997-55-7
N-Acetyl-L-aspartic acid
| Conditions | Yield |
|---|---|
|
L-Aspartic acid bis(trimethylsilyl) ester; acetyl chloride;
With
(benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate;
In
dichloromethane;
at 20 ℃;
With
methanol;
In
dichloromethane;
at 20 ℃;
|
997-55-7 Upstream products
-
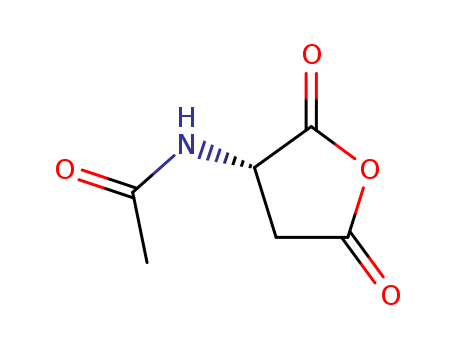
41148-79-2
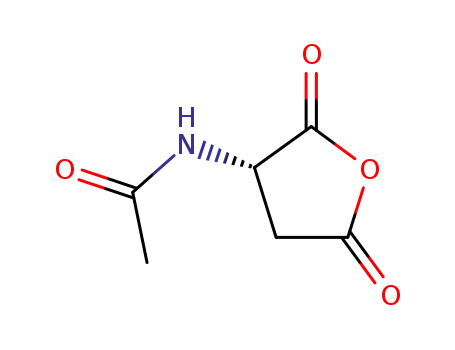
(S)-N-acetyl-L-aspartic anhydride
-
56-84-8
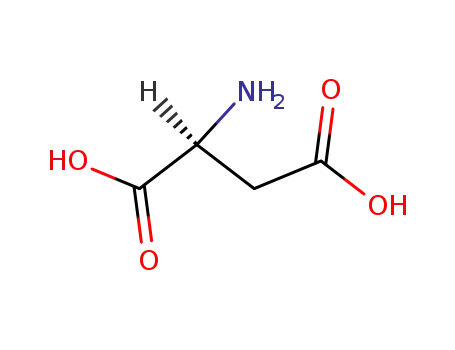
L-Aspartic acid
-
108-24-7
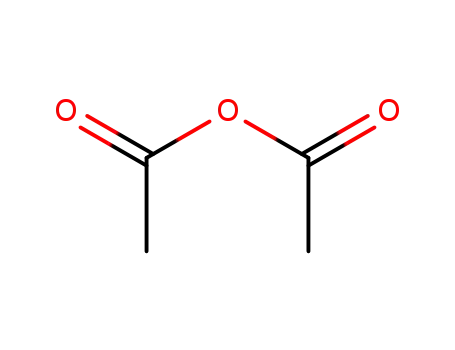
acetic anhydride
-
5269-42-1

L-Aspartic acid bis(trimethylsilyl) ester
997-55-7 Downstream products
-
41148-79-2

(S)-N-acetyl-L-aspartic anhydride
-
56-84-8

L-Aspartic acid