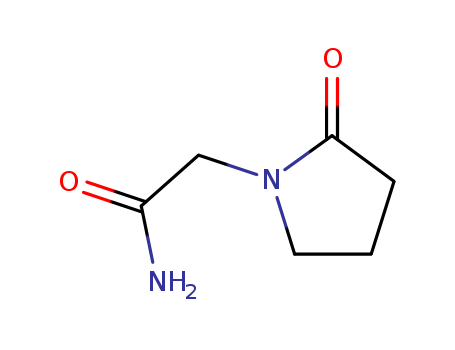
Piracetam
Piracetam
Factory Sells Best Quality Piracetam 7491-74-9 with USP
- Molecular Formula:C6H10N2O2
- Molecular Weight:142.158
- Vapor Pressure:7.06E-07mmHg at 25°C
- Melting Point:151-152 °C
- Refractive Index:1.523
- Boiling Point:408.3 °C at 760 mmHg
- PKA:15.67±0.40(Predicted)
- Flash Point:200.8 °C
- PSA:63.40000
- Density:1.239 g/cm3
- LogP:-0.26770
Piracetam(Cas 7491-74-9) Usage
|
Indication |
It is indicated in the case of adult patients suffering from myoclonus of cortical origin, irrespective of aetiology, and should be used in combination with other anti-myoclonic therapies. |
|
Pharmacological effects |
Neuronal effects Piracetam has important effects on neurotransmission that are not limited to any one type of neurotransmitter. It has been shown to influence cholinergic[3,4], serotoninergic[5], noradrenergic[6], and glutamatergic[7] systems. The modulation of these systems by piracetam does not result from direct receptor agonism or antagonism(piracetam has no affinity for these receptors; Ki > 10 μM)[8]. Instead, piracetam appears to increase the number of postsynaptic receptors and or restore the function of these receptors. The effect of piracetam on cholinergic and glutamatergic systems is likely to be particularly relevant to its clinical benefit in cognitive disorders, given the increasing evidence that dysfunction in these systems may be related to cognitive decline[9,10]. Preclinical studies have shown that piracetam appears to offer neuroprotective benefits. This is consistent with the suggestion that interactions between piracetam and membrane lipids may decrease the risk of membrane fusion[11]. Piracetam has been shown to reduce the incidence of animal death following barbiturate overdose, and to protect against morphological changes related to long-term alcohol use[12]. The anticonvulsant action of piracetam has also been documented in animal studies. Administration of piracetam prior to a convulsant stimulus reduces seizure severity in rats prone to audiogenic attacks[13]. Vascular effects Studies suggest that piracetam exerts a number of effects on erythrocytes, such as decreased adhesion to endothelium[14]. These effects are likely to facilitate movement of erythrocytes through the circulation. Studies have also indicated that piracetam exerts an effect on blood vessels. For example, in vitro, 2 mg/kg piracetam decreased the time taken for rabbit pial vessels to return to normal diameter following a period of induced arteriolar spasm(10.4 vs. 5.1 min for 0.02 and 2 mg/kg of piracetam, respectively)[15]. Piracetam may also influence blood coagulation. In healthy humans, a single dose of piracetam(3.2 to 9.6 g)?reduced plasma levels of fibrinogen and von Willebrand factor in a dose-dependent manner by up to 40%[16]. Enhanced cerebral blood flow has been reported following piracetam treatment in hypotensive cats and in humans with acute cerebral ischemia[17,18]. Additionally, piracetam appears to influence microcirculation at the peripheral level. Following treatment with piracetam, renal blood flow was significantly greater in ischemically damaged rat kidneys relative to controls[19], and, in a separate experiment, blood flow significantly increased in the cochlea of guinea pigs without any marked change in blood pressure[20]. |
|
Pharmacokinetics |
Piracetam is rapidly absorbed. Following oral administration, peak plasma concentrations in fasting subjects are achieved in approximately 30 min[22]. Following a single oral dose of 3.2 g, peak concentration is typically 84 ug/mL. Oral formulations of piracetam are extensively absorbed with a bioavailability close to 100%[22]. No metabolites of piracetam have yet been discovered and the drug is excreted unchanged in the urine by glomerular filtration[21]. While food does not affect the extent of absorption of piracetam, it does decrease the maximal plasma concentration of the drug by 17% and prolong Tmax to 1.5 h. Piracetam crosses blood–brain and placental barriers and is found in all tissues, except adipose tissue. The uptake into the brain is less rapid than into the circulation, and, at nearly 8 h, half-life in cerebrospinal fluid is longer than in plasma(about 5 h)[21]. |
|
Mode of action |
Although piracetam is a derivative of GABA, its mechanism of action appears to be unrelated to the properties of this neurotransmitter. While the exact mode of action of piracetam is a matter of debate, there is increasing evidence that its underlying effect is to restore cell membrane fluidity. Cell membranes comprise a bilayer of lipid molecules interspersed with protein molecules. These membranes are fluid structures in which the molecules comprising the membrane can diffuse while maintaining this overall arrangement. Membrane fluidity is believed to be important for a number of activities including membrane transport, enzyme activity, chemical secretion, and receptor binding and stimulation[23,24]. Piracetam can have direct interaction with the membrane. The resultant mobile drug–lipid complexes are thought to induce the reorganization of lipids, which may influence membrane function and fluidity[25]. Studies have also demonstrated that piracetam influences membrane fluidity, particularly when normal fluidity is compromised, as is often seen during aging[26]. Several in vitro studies have assessed fluidity during piracetam treatment using anisotropy of membrane-bound DPH[1,6-diphenyl-1,3,5-hexatriene]. Incubation with piracetam restored fluidity in brain membranes of elderly mice with diminished fluidity but had no effect on brain membranes of younger mice with normal fluidity[27]. Similarly, in other studies using in vitro anisotropy techniques, fluidity was restored in the membranes of aged rat and aged human brains following incubation with piracetam[27]. Similar effects were observed in hippocampal membranes from patients with Alzheimer’s disease[28]. This improvement of fluidity coincided with significantly improved avoidance learning[27]. No effect of piracetam on learning or membrane fluidity was found in young rats receiving piracetam. Through restored membrane fluidity, piracetam can promote restored neurotransmission such as glutamatergic and cholinergic systems, enhances neuroplasticity and mediates neuroprotective and anticonvulsant effects at the neuronal level. It has also been found that piracetam can also improves the fluidity of platelet membranes and decreases adhesion of erythrocytes to cell wall as well as reduces vasospasm which in turn improves microcirculation including cerebral and renal blood flow[23,24]. |
|
Toxicity |
Piracetam is remarkably well tolerated. In preclinical trials, no irreversible toxicity was reported in mice, rats or dogs receiving single oral doses of up to 10 g/kg. In a pooled analysis of 91 double-blind, placebo-controlled studies, hyperkinesia, weight gain, nervousness, somnolence, depression and asthenia were slightly increased with piracetam, although the incidence of each of these events was less than 2%. |
|
Contradictions and drug interactions |
Due to its renal clearance, piracetam dose should be adjusted in patients with renal insufficiency and the drug is contraindicated in patients with end-stage renal disease. Piracetam should not be prescribed to patients with cerebral hemorrhage. While reproductive studies in animals have not identified any risk to the fetus, studies in humans have not been conducted and so the use of piracetam in pregnant or lactating women should be avoided. Piracetam is neither metabolized by the liver nor bound to plasma albumin. The potential for drug–drug interactions is, therefore, low. Although piracetam enhances the anticonvulsant effects of carbamazepine, no interactions with sodium valproate have been reported[29]. There are no known interactions of piracetam with any other drugs. |
|
Side effects |
Despite that piracetam is regarded as a relatively safe nootropic, some users may still experience side effects and adverse reactions[30]. The severity and number of side effects experienced from piracetam is subject to significant individual variation. One user may not perceive any side effects, while another may notice severe adverse reactions. Of all those reported piracetam side effects, the most common include: nervousness, increased body movements(hyperkinesia), and weight gain. Some rare cases may also include agitation, anxiety, brain fog, cognitive impairment, depression, fatigue, hemocrit/hemoglobin levels change, headaches, hyperkinesia, insomnia, irritability, libido increase, lightheadedness, muscle spasms, nausea, restlessness, shakiness, sleep disturbances, speech impairment, somnolence, sweating, visual change and weakness. |
|
Manufacturing Process |
2-Pyrrolidone is first reacted with sodium hydride, then with ethyl chloroacetate to give ethyl 2-oxo-1-pyrrolidine acetate.A solution of 0.3 mol of ethyl 2-oxo-1-pyrrolidine acetate in 300 ml of methanol, saturated with ammonia at 20° to 30°C, is heated at 40° to 50°C for 5 hours, while continuously introducing ammonia. The reaction mixture is evaporated to dryness and the residue recrystallized from isopropanol. 2-Oxo1-pyrrolidineacetamide is obtained in a yield of 86%. MP 151.5° to 152.5°C. |
|
Therapeutic Function |
Psychotropic |
|
Biological Activity |
Nootropic that displays cognitive enhancing properties. Proposed to enhance neurotransmission via modulation of ion flux; potentiates Na + influx through AMPA receptors. Facilitates efficiency of cholinergic neurotransmission at muscarinic receptors. |
|
Biochem/physiol Actions |
Piracetam (2-oxo-1-pyrrolidinyl-acetamide) is a nootropic drug, which enhances memory and facilitates learning. Piracetam also acts as a vasodilator and improves blood flow. It has a potential to treat cognitive impairment in aging, brain injury, memory and balance problems. In addition, piracetam is also used to treat peripheral vascular disease. |
|
Safety Profile |
Mildly toxic by ingestion. Whenheated to decomposition it emits toxic fumes of NOx. |
|
Metabolism |
Potentially hazardous interactions with other drugs None known |
|
Purification Methods |
This typical nootropic (Alzheimer) drug modulates Na flux in AMPA receptors and is purified by recrystallisation from isoPrOH. [Gouilaev & Senning Brain Research Rev 19 180 1994.] |
|
Overview |
Piracetam is a cyclic derivative of the neurotransmitter gamma-aminobutyric acid(GABA), originally marketed in 1971 by UCB Pharma. It was the first “nootropic” drug[1], an agent that acts on cognitive function without causing sedation or stimulation. While the mechanisms of action of piracetam have yet to be fully elucidated, it influences neuronal and vascular functions. Furthermore, vascular effects are peripheral as well as central, meaning that the clinical benefit of piracetam goes beyond its nootropic features. Indeed, piracetam is now indicated for use in vertigo, dyslexia, cortical myoclonus and sickle cell anemia in addition to age-related cognitive disorders. Piracetam has been available for over 40 years. Its efficacy has been recorded in cognitive disorders and dementia, vertigo, cortical myoclonus, dyslexia, and sickle cell anemia, however, the clinical application in these conditions is not yet established. Piracetam also has effects on the vascular system through reducing erythrocyte adhesion to vascular endothelium, hinder vasospasm and facilitate microcirculation[2]. Figure 1 The chemical structure of piracetam ; |
InChI:InChI=1/C6H10N2O2/c7-5(9)4-8-3-1-2-6(8)10/h1-4H2,(H2,7,9)
7491-74-9 Relevant articles
Aerobic oxidation of primary amines to amides catalyzed by an annulated mesoionic carbene (MIC) stabilized Ru complex
Yadav, Suman,Reshi, Noor U Din,Pal, Saikat,Bera, Jitendra K.
, p. 7018 - 7028 (2021/11/17)
Catalytic aerobic oxidation of primary a...
TYK2 INHIBITORS AND USES THEREOF
-
Paragraph 00766-00767, (2020/06/19)
The present invention provides compounds...
Process for synthesizing piracetam (by machine translation)
-
Paragraph 0051-0065, (2019/07/16)
The invention belongs to the field, and ...
Diversity-Oriented Synthesis of Heterocycles: Al(OTf)3-Promoted Cascade Cyclization and Ionic Hydrogenation
Liu, Tianqi,Jia, Wenqiang,Xi, Qiumu,Chen, Yonghui,Wang, Xiaojian,Yin, Dali
, p. 1387 - 1393 (2018/02/10)
An efficient and facile method has been ...
7491-74-9 Process route
-
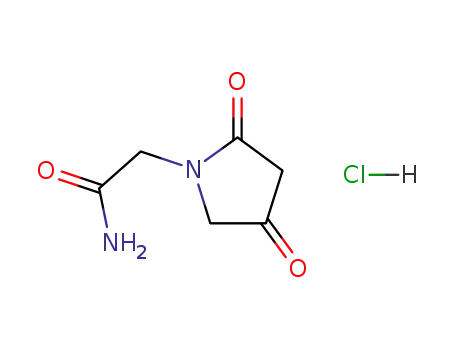
- 142352-10-1
(2,4-dioxopyrrolidin-1-yl)acetamide hydrochloride

-
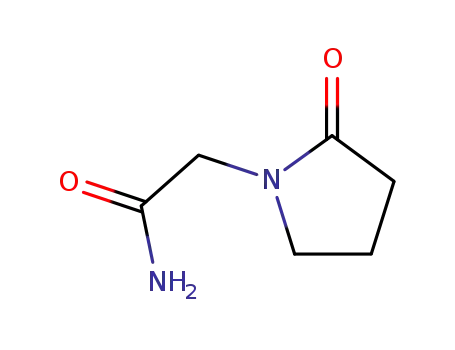
- 7491-74-9
2-oxo-1-pyrrolidine acetamide

-
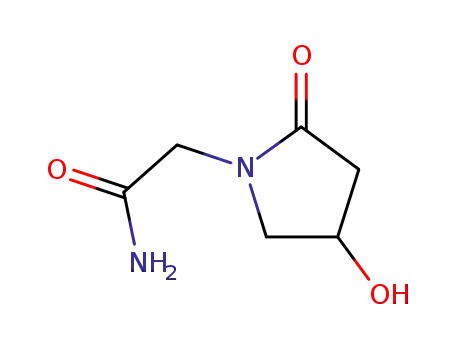
- 68252-28-8,68567-97-5,88929-35-5,62613-82-5
oxiracetam

-
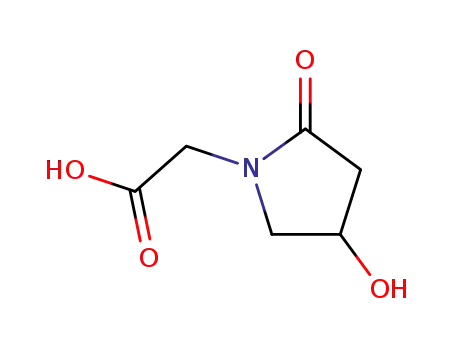
- 77191-37-8,99437-11-3
4-hydroxy-2-oxo-1-pyrrolidine acetic acid

-
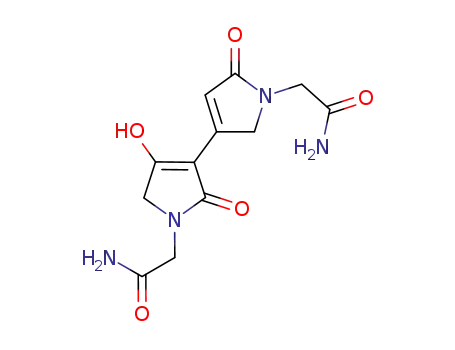
- 105240-61-7
3-(1-(carbamoylmethyl)-2-oxo-3-pyrrolin-4-yl)-4-hydroxy-2-oxo-3-pyrroline-1-acetamide
| Conditions | Yield |
|---|---|
|
With hydrogen; Ru-carbon; In water; for 20h; under 18751.5 Torr; Ambient temperature;
|
24% 45% |
-
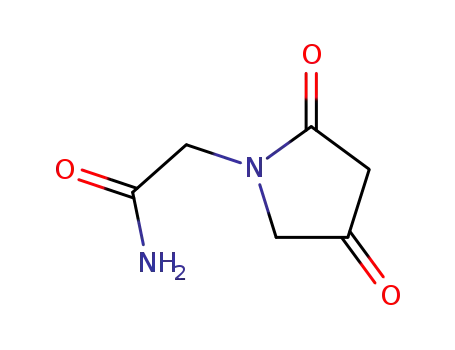
- 85614-54-6
2-(2,4-dioxopyrrolidine-1-yl)acetamide

-

- 7491-74-9
2-oxo-1-pyrrolidine acetamide

-

- 68252-28-8,68567-97-5,88929-35-5,62613-82-5
oxiracetam

-

- 105240-61-7
3-(1-(carbamoylmethyl)-2-oxo-3-pyrrolin-4-yl)-4-hydroxy-2-oxo-3-pyrroline-1-acetamide
| Conditions | Yield |
|---|---|
|
With methanesulfonic acid; hydrogen; Pt/Al2O3; for 14h; under 11250.9 Torr; Yield given. Yields of byproduct given;
|
7491-74-9 Upstream products
-
61516-73-2
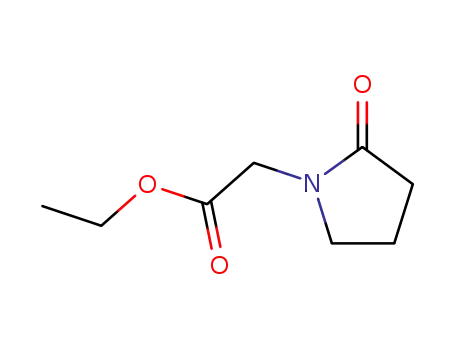
2-(oxo-pyrrolidin-1-yl)-acetic acid ethyl ester
-
64026-51-3
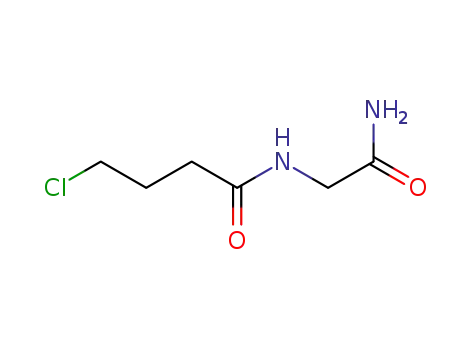
2-(4-chlorobutyramido)acetamide
-
59776-88-4
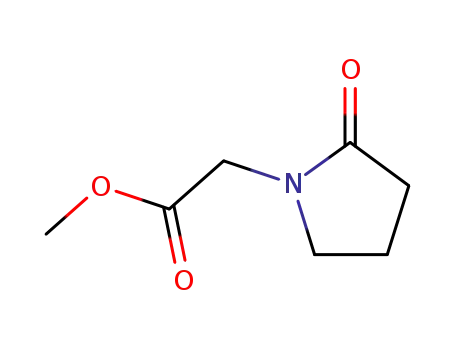
methyl-2-oxopyrrolidine-1-acetate
-
120-20-7
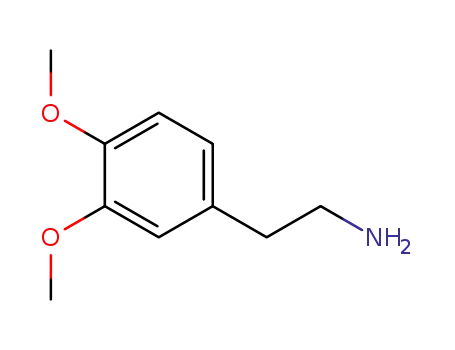
2-(3,4-dimethoxyphenyl)-ethylamine
7491-74-9 Downstream products
-
57275-83-9
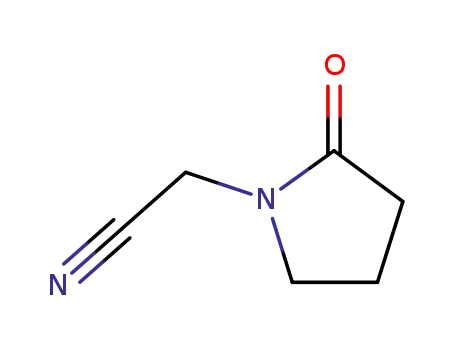
nitrile of 2-oxopyrrolidine-1-acetic acid
-
117947-07-6
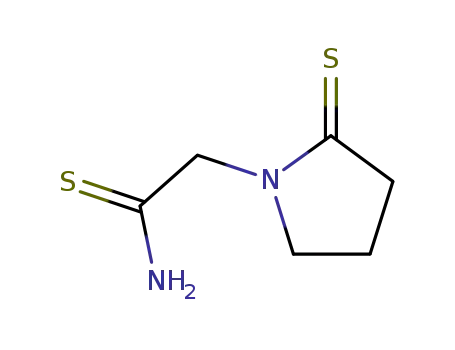
1-thiocarbamoylmethylpyrrolidine-2-thione
-
91417-91-3
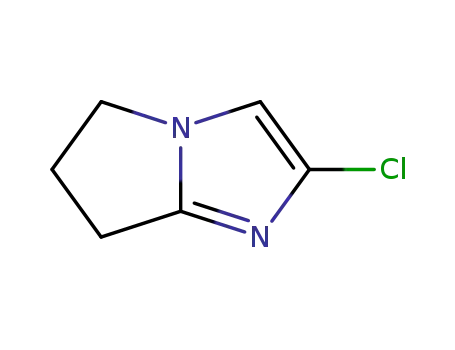
2-Chloro-5H,6,7-dihydropyrrolo<1,2-a>imidazole
-
91417-95-7
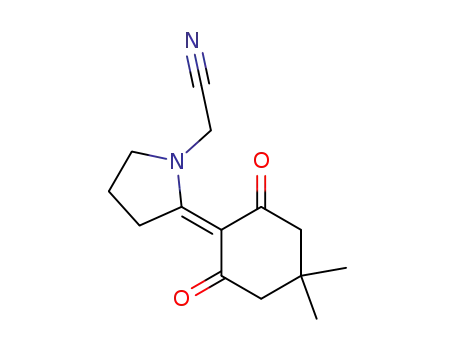
1,3-Dioxo-2-(N-cyanomethyl-2-pyrrolidinene)-5,5-dimethylcyclohexane