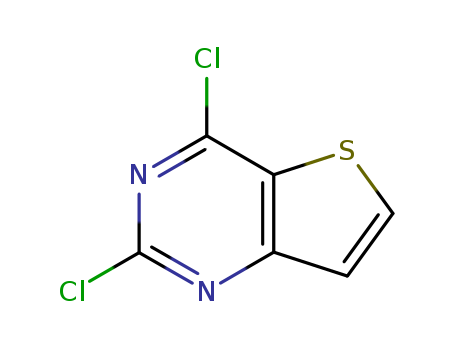
Thieno[3,2-d]pyrimidine, 2,4-dichloro-
Thieno[3,2-d]pyrimidine, 2,4-dichloro-
Reliable factory customized supply Thieno[3,2-d]pyrimidine, 2,4-dichloro- 16234-14-3
- Molecular Formula:C6H2Cl2N2S
- Molecular Weight:205.067
- Melting Point:136.0 to 140.0 °C
- Boiling Point:274.804 °C at 760 mmHg
- PKA:-0.72±0.40(Predicted)
- Flash Point:119.997 °C
- PSA:54.02000
- Density:1.662 g/cm3
- LogP:2.99810
16234-14-3 Relevant articles
Discovery of novel and potent PARP/PI3K dual inhibitors for the treatment of cancer
Wu, Zhengyang,Bai, Ying,Jin, Jiaming,Jiang, Teng,Shen, Hui,Ju, Qiurong,Zhu, Qihua,Xu, Yungen
, (2021/03/19)
PARP inhibitors have achieved great succ...
PARP-1/PI3K double-target inhibitor or pharmaceutically acceptable salt thereof, preparation method and application thereof
-
Paragraph 0157; 0162-0164; 0191; 0195-0197, (2021/07/01)
The invention discloses a PARP-1/PI3K do...
Design, synthesis and biological evaluation of novel 2,4-bismorpholinothieno[3,2-d]pyrimidine and 2-morpholinothieno[3,2-d]pyrimidinone derivatives as potent antitumor agents
Ye, Tianyu,Han, Yufei,Wang, Ruxin,Yan, Pingzhen,Chen, Shaowei,Hou, Yunlei,Zhao, Yanfang
, (2020/04/15)
To develop novel therapeutic agents with...
COMBINATION THERAPY WITH A PHOSPHOINOSITIDE 3-KINASE INHIBITOR WITH A ZINC BINDING MOIETY
-
Paragraph 0087; 0103, (2020/04/09)
The invention provides a method of treat...
16234-14-3 Process route
-
![1H-thieno[3,2-d]pyrimidine-2,4-dione](/upload/2024/8/9da93609-d9a0-4cc0-97ce-68066f4bf6d2.png)
-
16233-51-5
1H-thieno[3,2-d]pyrimidine-2,4-dione

-
![2,4-dichlorothieno[3,2-d]pyrimidine](/upload/2024/8/5e188413-3a4a-4bf2-b18f-3b8c4b2c81b8.png)
-
16234-14-3
2,4-dichlorothieno[3,2-d]pyrimidine
| Conditions | Yield |
|---|---|
|
With
N-ethyl-N,N-diisopropylamine; trichlorophosphate;
for 2h;
Heating / reflux;
|
100% |
|
With
N,N-dimethyl-formamide; trichlorophosphate;
at 110 ℃;
for 2h;
|
95.4% |
|
With
N,N-diethylaniline; trichlorophosphate;
at 105 ℃;
for 16h;
|
92% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
In
acetonitrile;
at 20 - 85 ℃;
for 26h;
Inert atmosphere;
Large scale;
|
89% |
|
With
N-ethyl-N,N-diisopropylamine; trichlorophosphate;
for 2h;
Reflux;
|
87.3% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
In
acetonitrile;
at 0 - 85 ℃;
for 48h;
|
84% |
|
With
trichlorophosphate;
at 80 ℃;
for 8h;
|
84.7% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
In
acetonitrile;
at 20 - 85 ℃;
for 24h;
Cooling;
|
83% |
|
With
trichlorophosphate;
In
N,N-dimethyl-aniline; acetonitrile;
at 15 - 85 ℃;
for 24h;
|
83% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
In
acetonitrile;
at 85 ℃;
for 24h;
|
83% |
|
With
trichlorophosphate;
at 105 - 110 ℃;
for 16h;
Inert atmosphere;
|
82% |
|
With
trichlorophosphate;
for 16h;
Inert atmosphere;
Reflux;
|
82% |
|
With
triethylamine; trichlorophosphate;
at 0 ℃;
for 8h;
Reflux;
|
81.7% |
|
With
trichlorophosphate;
for 8h;
Reflux;
|
81.4% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
In
acetonitrile;
at 50 - 85 ℃;
Large scale;
|
79.6% |
|
With
trichlorophosphate;
for 6h;
Heating / reflux;
|
75% |
|
With
trichlorophosphate;
for 6h;
Heating / reflux;
|
75% |
|
With
trichlorophosphate;
for 6h;
Heating / reflux;
|
75% |
|
With
trichlorophosphate;
for 6h;
Heating / reflux;
|
75% |
|
1H-thieno[3,2-d]pyrimidine-2,4-dione;
With
trichlorophosphate;
for 6h;
Heating / reflux;
With
water;
|
75% |
|
With
trichlorophosphate;
In
acetonitrile;
for 24h;
Heating / reflux;
|
75% |
|
With
trichlorophosphate;
|
75% |
|
With
trichlorophosphate;
for 6h;
Reflux;
|
75% |
|
With
trichlorophosphate;
for 6h;
Reflux;
|
75% |
|
With
trichlorophosphate;
In
acetonitrile;
for 24h;
Heating / reflux;
|
75% |
|
With
trichlorophosphate;
for 6h;
Heating / reflux;
|
75% |
|
With
trichlorophosphate;
for 6h;
Heating / reflux;
|
75% |
|
With
trichlorophosphate;
for 10h;
Reflux;
|
74% |
|
With
trichlorophosphate;
for 5h;
Reflux;
|
74% |
|
With
trichlorophosphate;
for 10h;
Reflux;
|
74% |
|
With
trichlorophosphate;
at 110 ℃;
for 5h;
|
74% |
|
With
trichlorophosphate;
for 10h;
Reflux;
|
74% |
|
With
trichlorophosphate;
at 200 ℃;
for 3h;
|
73.3% |
|
With
trichlorophosphate;
for 8h;
Inert atmosphere;
Heating;
|
73% |
|
With
N,N-dimethyl-formamide; trichlorophosphate;
for 8h;
Reflux;
|
72% |
|
With
N,N-dimethyl-formamide; trichlorophosphate;
for 8h;
Reflux;
|
72% |
|
With
trichlorophosphate;
In
N,N-dimethyl-formamide;
for 8h;
Reflux;
|
72% |
|
With
trichlorophosphate;
for 10h;
Reflux;
|
70% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
for 16h;
Reflux;
|
67% |
|
With
trichlorophosphate;
for 8h;
Reflux;
|
67% |
|
With
trichlorophosphate;
at 106 ℃;
for 3h;
Inert atmosphere;
|
41% |
|
With
phosphorus pentachloride; trichlorophosphate;
at 135 ℃;
for 5h;
|
36% |
|
With
trichlorophosphate;
at 116 ℃;
for 5h;
|
28% |
|
With
2,3-Dimethylaniline; trichlorophosphate;
In
diethyl ether; water;
|
|
|
With
N,N-diethylaniline; trichlorophosphate;
at 100 ℃;
for 12h;
|
|
|
With
N,N-dimethyl-formamide; trichlorophosphate;
for 8h;
Reflux;
|
|
|
With
trichlorophosphate;
for 6h;
Reflux;
|
16.9 g |
|
With
N,N-dimethyl-formamide; trichlorophosphate;
Reflux;
|
|
|
With
trichlorophosphate;
at 100 ℃;
for 10h;
|
|
|
With
phosphorus pentachloride; trichlorophosphate;
at 120 ℃;
for 6h;
Inert atmosphere;
|
|
|
With
trichlorophosphate;
at 100 ℃;
for 10h;
|
|
|
With
N,N-dimethyl-formamide; trichlorophosphate;
for 8h;
Reflux;
|
|
|
With
trichlorophosphate;
for 8h;
Reflux;
|
-
![2,4-dihydroxythieno[3,2-d]pyrimidine](/upload/2024/8/86890e10-2ed0-4f38-b5bc-5084a99c4b6a.png)
-
16233-51-5
2,4-dihydroxythieno[3,2-d]pyrimidine

-
![2,4-dichlorothieno[3,2-d]pyrimidine](/upload/2024/8/5e188413-3a4a-4bf2-b18f-3b8c4b2c81b8.png)
-
16234-14-3
2,4-dichlorothieno[3,2-d]pyrimidine
| Conditions | Yield |
|---|---|
|
With
trichlorophosphate;
at 100 ℃;
Product distribution / selectivity;
|
100% |
|
With
trichlorophosphate;
at 120 ℃;
|
100% |
|
With
trichlorophosphate;
In
acetonitrile;
for 4h;
Reflux;
|
95% |
|
With
trichlorophosphate;
for 14h;
Heating / reflux;
|
93% |
|
With
trichlorophosphate;
for 14h;
Heating / reflux;
|
93% |
|
With
trichlorophosphate;
In
acetonitrile;
at 90 ℃;
for 8h;
|
81.4% |
|
With
1-methyl-pyrrolidin-2-one; trichlorophosphate;
In
toluene;
Reflux;
Inert atmosphere;
|
80% |
|
With
1-methyl-pyrrolidin-2-one; trichlorophosphate;
In
toluene;
for 16h;
Reflux;
|
80% |
|
With
trichlorophosphate;
In
acetonitrile;
for 48h;
Reflux;
|
79% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
In
acetonitrile;
at 80 - 85 ℃;
for 72h;
|
79% |
|
With
trichlorophosphate;
In
N,N-dimethyl-formamide;
at 120 ℃;
for 3h;
|
79.6% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
for 14h;
Heating / reflux;
|
74.4% |
|
With
trichlorophosphate;
for 10h;
Reflux;
|
70.5% |
|
With
trichlorophosphate;
for 10h;
Reflux;
|
70.5% |
|
With
trichlorophosphate;
for 12h;
Reflux;
|
69% |
|
With
N,N-dimethyl-formamide; trichlorophosphate;
for 12h;
Reflux;
|
69.8% |
|
With
P,P-dichlorophenylphosphine oxide;
at 170 ℃;
for 2h;
|
66% |
|
With
P,P-dichlorophenylphosphine oxide;
at 170 ℃;
for 2h;
|
66% |
|
With
P,P-dichlorophenylphosphine oxide;
at 180 ℃;
for 4h;
|
60% |
|
With
trichlorophosphate;
In
N,N-dimethyl-aniline;
for 3h;
|
51.2% |
|
With
trichlorophosphate;
|
16234-14-3 Upstream products
-
16233-51-5
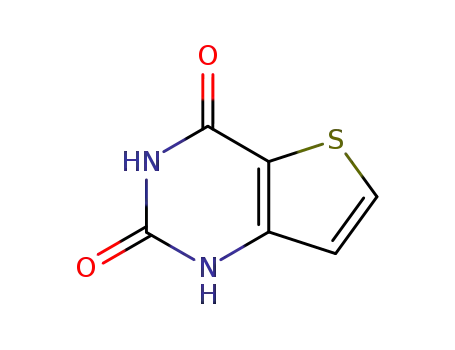
1H-thieno[3,2-d]pyrimidine-2,4-dione
-
16233-51-5
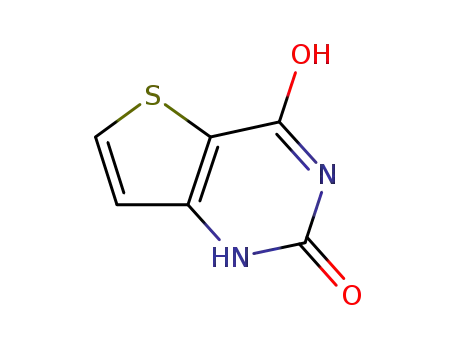
thienoyleneurea
-
16233-51-5
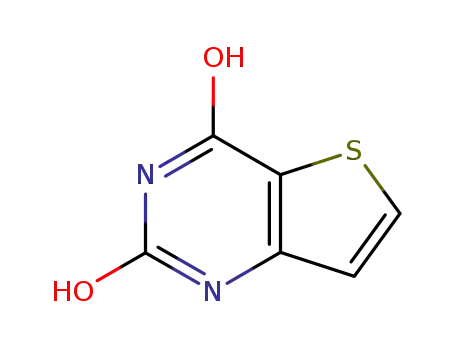
2,4-dihydroxythieno[3,2-d]pyrimidine
-
22288-78-4
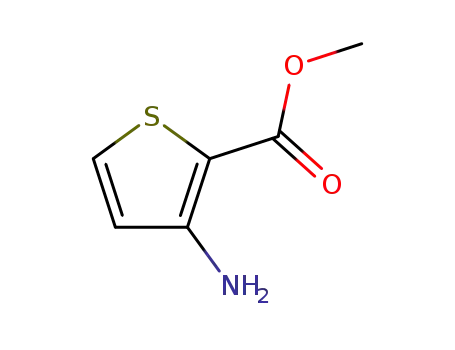
Methyl 3-aminothiophene-2-carboxylate
16234-14-3 Downstream products
-
147972-18-7
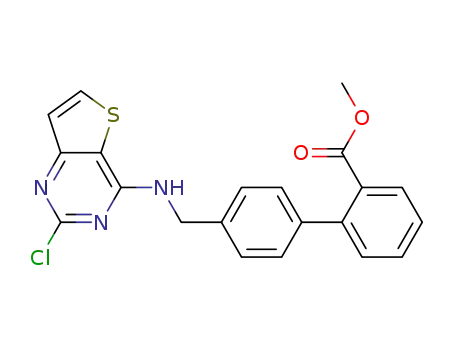
4'-[[[2-chlorothieno-[3,2-d]pyrimidin-4-yL]amino]methyl][1,1'-biphenyl]-2-carboxylic acid methyl ester
-
152943-20-9
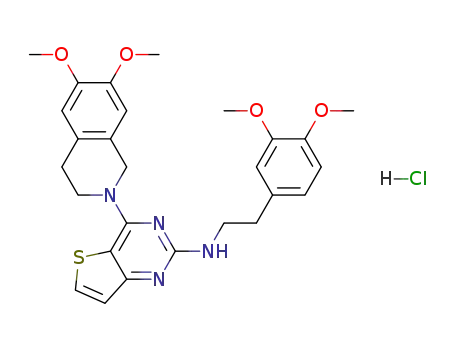
2-(3,4-Dimethoxyphenethylamino)-4-(1,2,3,4-tetrahydro-6,7-dimethoxyisoquinol-2-yl)thieno[3,2-d]pyrimidine hydrochloride
-
1024603-85-7
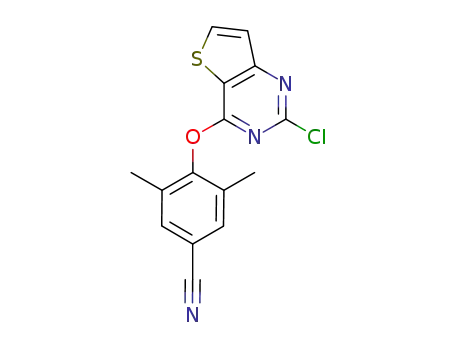
4-((2-chlorothiophene[3,2-d]pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile
-
1024603-87-9
2-chloro-N-mesitylthieno[3,2-d]pyrimidin-4-amine