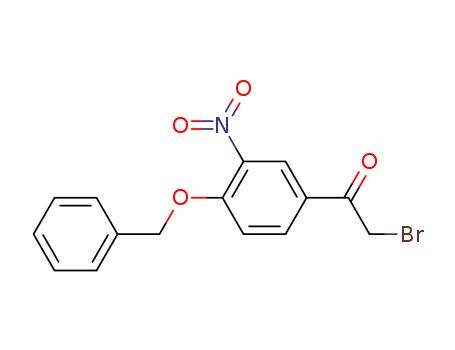
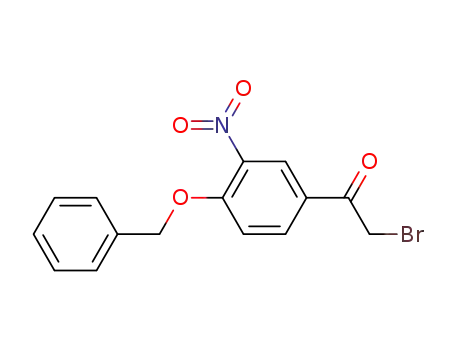
2-Bromo-4'-benzyloxy-3'-nitroacetophenone
2-Bromo-4'-benzyloxy-3'-nitroacetophenone
Export Top Purity 2-Bromo-4'-benzyloxy-3'-nitroacetophenone 43229-01-2 In Stock
- Molecular Formula:C15H12BrNO4
- Molecular Weight:350.169
- Vapor Pressure:0mmHg at 25°C
- Melting Point:135-137 °C(Solv: ethanol (64-17-5))
- Refractive Index:1.627
- Boiling Point:465.239 °C at 760 mmHg
- Flash Point:235.168 °C
- PSA:72.12000
- Density:1.518 g/cm3
- LogP:4.27460
2-Bromo-4'-Benzyloxy-3'-nitroacetophenone(Cas 43229-01-2) Usage
InChI:InChI=1/C15H12BrNO4/c16-9-14(18)12-6-7-15(13(8-12)17(19)20)21-10-11-4-2-1-3-5-11/h1-8H,9-10H2
43229-01-2 Relevant articles
Preparation method of 3-nitro-4-benzyloxy-2-bromoacetophenone
-
Paragraph 0016; 0020, (2018/09/08)
The invention relates to a preparation m...
Benzylether fumaric acid luck not Trow an important intermediate for the synthesis of compound
-
Paragraph 0059; 0062, (2017/01/23)
The invention discloses a synthetic meth...
Design, synthesis and evaluation of dual pharmacology β2- adrenoceptor agonists and PDE4 inhibitors
Huang, Ling,Shan, Wenjun,Zhou, Qi,Xie, Jiaxing,Lai, Kefang,Li, Xingshu
, p. 249 - 253 (2014/01/17)
A novel series of formoterol-phthalazino...
Dual β2-adrenoceptor agonists-PDE4 inhibitors for the treatment of asthma and COPD
Shan, Wen-Jun,Huang, Ling,Zhou, Qi,Jiang, Huai-Lei,Luo, Zong-Hua,Lai, Ke-Fang,Li, Xing-Shu
, p. 1523 - 1526 (2012/04/04)
We designed and synthesized a novel clas...
43229-01-2 Process route
-
-
6322-56-1
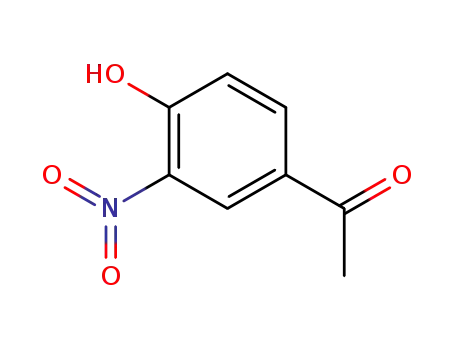
4'-hydroxy-3'-nitroacetophenone

-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: TBAI, K2CO3 / acetone
2: Br2 / CHCl3
With
bromine; tetra-(n-butyl)ammonium iodide; potassium carbonate;
In
chloroform; acetone;
|
|
|
Multi-step reaction with 2 steps
1.1: N-ethyl-N,N-diisopropylamine / acetonitrile / 20 °C
1.2: 3 h / 20 °C / Reflux
2.1: tetra-N-butylammonium tribromide / tetrahydrofuran; methanol / 4 h
With
tetra-N-butylammonium tribromide; N-ethyl-N,N-diisopropylamine;
In
tetrahydrofuran; methanol; acetonitrile;
|
|
|
Multi-step reaction with 2 steps
1.1: potassium carbonate / water; acetone / 20 °C
1.2: 17 h / Reflux
2.1: bromine / acetic acid / 20 °C
With
bromine; potassium carbonate;
In
water; acetic acid; acetone;
|
|
|
Multi-step reaction with 2 steps
1: potassium carbonate / acetone; water / 60 °C
2: bromine / acetic acid / 20 °C
With
bromine; potassium carbonate;
In
water; acetic acid; acetone;
|
|
|
Multi-step reaction with 2 steps
1: potassium carbonate; sodium iodide / acetone / 66 h / Heating / reflux
2: phenyltrimethylammonium tribromide / tetrahydrofuran / 12 h / 20 °C
With
phenyltrimethylammonium tribromide; potassium carbonate; sodium iodide;
In
tetrahydrofuran; acetone;
|
-
-
99-93-4
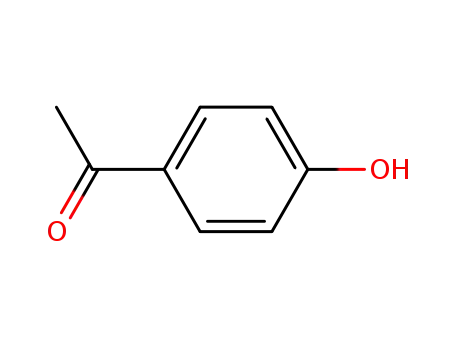
4-Hydroxyacetophenone

-

-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: HNO3 / acetic acid / 0 °C
2: TBAI, K2CO3 / acetone
3: Br2 / CHCl3
With
bromine; nitric acid; tetra-(n-butyl)ammonium iodide; potassium carbonate;
In
chloroform; acetic acid; acetone;
|
|
|
Multi-step reaction with 3 steps
1: HNO3 / acetic acid / -0.1 °C
2: TBAI, K2CO3 / acetone
3: Br2 / CHCl3
With
bromine; nitric acid; tetra-(n-butyl)ammonium iodide; potassium carbonate;
In
chloroform; acetic acid; acetone;
|
43229-01-2 Upstream products
-
14347-05-8
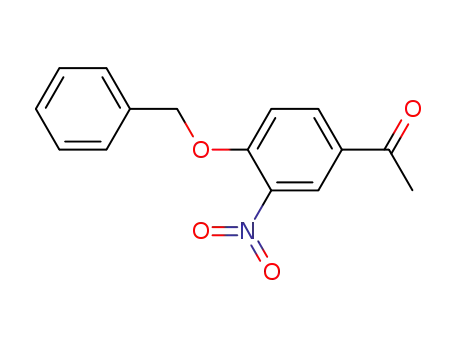
4-benzyloxy-3-nitroacetophenone
-
6322-56-1

4'-hydroxy-3'-nitroacetophenone
-
99-93-4

4-Hydroxyacetophenone
-
100-39-0
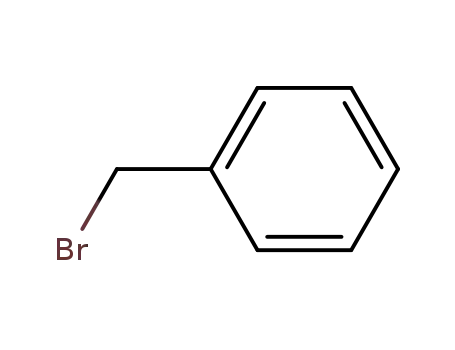
benzyl bromide
43229-01-2 Downstream products
-
51582-41-3
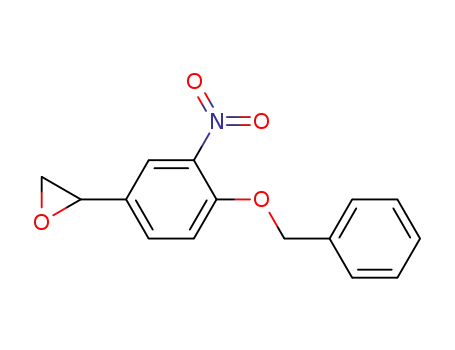
2-(3-benzyloxy-4-nitrophenyl)oxirane
-
299964-35-5
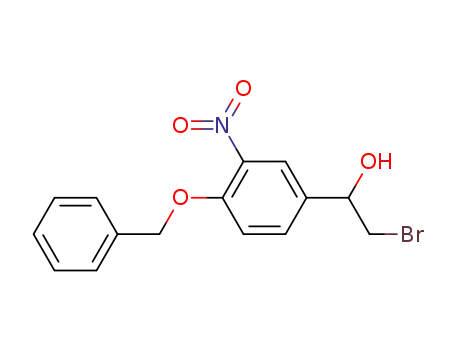
(+/-)-2-bromo-1-(4-benzyloxy-3-nitrophenyl)ethanol
-
173283-38-0
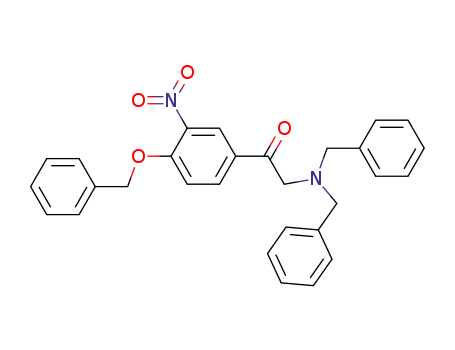
ω-(dibenzylamino)-4-benzyloxy-3-nitroacetophenone
-
193761-53-4
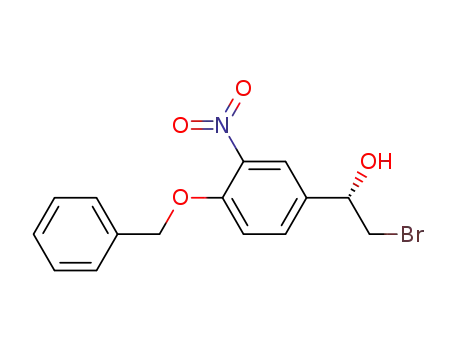
(S)-1-(4-(benzyloxy)-3-nitrophenyl)-2-bromoethanol