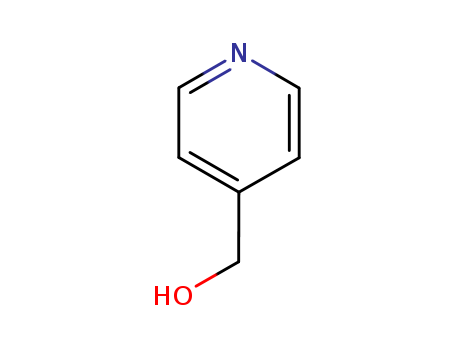
4-Pyridylcarbinol
4-Pyridylcarbinol
Export Top Purity 4-Pyridylcarbinol 586-95-8 In Stock
- Molecular Formula:C6H7NO
- Molecular Weight:109.128
- Appearance/Colour:white to yellow crystals, crystalline powder
- Vapor Pressure:0.00134mmHg at 25°C
- Melting Point:52-56 °C(lit.)
- Refractive Index:1.551
- Boiling Point:285.1 °C at 760 mmHg
- PKA:13.45±0.10(Predicted)
- Flash Point:98.9 °C
- PSA:33.12000
- Density:1.131 g/cm3
- LogP:0.57390
4-Pyridylcarbinol(Cas 586-95-8) Usage
InChI:InChI=1/C6H7NO/c8-5-6-1-3-7-4-2-6/h1-4,8H,5H2
586-95-8 Relevant articles
New heterocyclic mono- and bis(α-hydroxymethyl)phosphinic acids: Synthesis and CuII binding abilities
Olszewski, Tomasz Krzysztof,Galezowska, Joanna,Boduszek, Bogdan,Kozlowski, Henryk
, p. 3539 - 3546 (2007)
A simple and efficient method for the sy...
Novel quinolone-based potent and selective HDAC6 inhibitors: Synthesis, molecular modeling studies and biological investigation
Relitti, Nicola,Saraswati, A. Prasanth,Chemi, Giulia,Brindisi, Margherita,Brogi, Simone,Herp, Daniel,Schmidtkunz, Karin,Saccoccia, Fulvio,Ruberti, Giovina,Ulivieri, Cristina,Vanni, Francesca,Sarno, Federica,Altucci, Lucia,Lamponi, Stefania,Jung, Manfred,Gemma, Sandra,Butini, Stefania,Campiani, Giuseppe
, (2020/11/24)
In this work we describe the synthesis o...
Iron-catalyzed chemoselective hydride transfer reactions
Coufourier, Sébastien,Ndiaye, Daouda,Gaillard, Quentin Gaignard,Bettoni, Léo,Joly, Nicolas,Mbaye, Mbaye Diagne,Poater, Albert,Gaillard, Sylvain,Renaud, Jean-Luc
supporting information, (2021/06/07)
A Diaminocyclopentadienone iron tricarbo...
Homoleptic cobalt(II) phenoxyimine complexes for hydrosilylation of aldehydes and ketones without base activation of cobalt(II)
Hori, Momoko,Ishikawa, Ryuta,Koga, Yuji,Matsubara, Kouki,Mitsuyama, Tomoaki,Shin, Sayaka
, p. 1379 - 1387 (2021/05/29)
Air-stable, easy to prepare, homoleptic ...
Metal-Free Deoxygenation of Amine N-Oxides: Synthetic and Mechanistic Studies
Lecroq, William,Schleinitz, Jules,Billoue, Mallaury,Perfetto, Anna,Gaumont, Annie-Claude,Lalevée, Jacques,Ciofini, Ilaria,Grimaud, Laurence,Lakhdar, Sami
, p. 1237 - 1242 (2021/06/01)
We report herein an unprecedented combin...
586-95-8 Process route
-
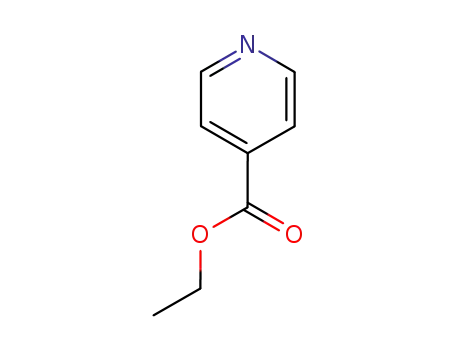
-
1570-45-2
isonicotinic acid ethylester

-
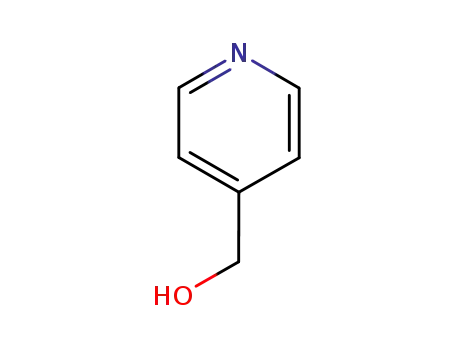
-
586-95-8
pyridine-4-methanol
| Conditions | Yield |
|---|---|
|
With
ethanol; potassium tert-butylate; C39H41FeMnN2O5P(1+)*Br(1-); 1-Methylnaphthalene;
In
tert-butyl alcohol;
at 100 ℃;
for 22h;
enantioselective reaction;
Inert atmosphere;
Schlenk technique;
|
83% |
|
With
hydrogen; [2-((diphenylphospino)methyl)-2-methyl-1,3-propanediyl]bis[diphenylphosphine];
tris(2,4-pentanedionato)ruthenium(III);
In
isopropyl alcohol;
at 150 ℃;
for 24h;
under 112511 Torr;
Product distribution / selectivity;
|
74.5% |
|
With
ethanol; calcium carbonate;
|
|
|
With
lithium aluminium tetrahydride; diethyl ether;
|
|
|
With
potassium borohydride; lithium chloride;
for 0.0666667h;
microwave irradiation;
|
97 % Chromat. |
|
With
C41H42Cl2N2P2Ru; hydrogen;
at 100 ℃;
for 25h;
|
|
|
With
C41H42Cl2N2P2Ru; potassium tert-butylate; hydrogen;
at 100 ℃;
for 25h;
|
|
|
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
at 0 ℃;
for 7h;
Reflux;
|
-
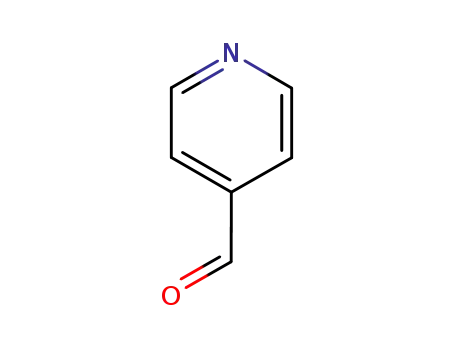
-
872-85-5
pyridine-4-carbaldehyde

-

-
586-95-8
pyridine-4-methanol
| Conditions | Yield |
|---|---|
|
With
magnesium(II) perchlorate; polymer-bound NADH (2a);
In
acetonitrile; benzene;
at 80 ℃;
for 120h;
Further byproducts given;
|
100% |
|
With
sodium tetrahydroborate; lithium perchlorate;
In
acetonitrile;
for 0.25h;
|
98% |
|
With
methanol; sodium tetrahydroborate;
for 0.5h;
Product distribution / selectivity;
Cooling with ice;
|
98% |
|
With
alumina; isopropyl alcohol;
at 180 ℃;
for 0.666667h;
under 11251.1 Torr;
Microwave irradiation;
Sealed tube;
|
98% |
|
With
isopropyl alcohol;
at 300 ℃;
for 3h;
|
95% |
|
With
borane-ammonia complex;
In
water;
at 20 ℃;
for 0.133333h;
chemoselective reaction;
|
91% |
|
With
sodium dithionite; sodium hydrogencarbonate;
In
water; isopropyl alcohol;
at 110 ℃;
for 2.66667h;
Flow reactor;
|
91% |
|
With
trimethylamine-N-oxide; sodium formate; C34H44FeN4O4(2+)*2I(1-);
In
water;
at 80 ℃;
for 24h;
Inert atmosphere;
Schlenk technique;
|
91% |
|
With
ammonium chloride; zinc;
In
tetrahydrofuran; water;
at 20 ℃;
for 0.333333h;
|
88% |
|
With
1-acetyl-2,3-dimethylimidazolidine;
In
methanol; acetonitrile;
for 3h;
Heating;
|
87% |
|
With
Ca(BH2S3)2;
In
tetrahydrofuran;
for 1.2h;
Heating;
|
86% |
|
With
magnesium(II) perchlorate; 1-acetyl-2,3-dimethyltetrahydropyrimidine;
In
methanol; acetonitrile;
at 50 ℃;
for 0.5h;
|
84% |
|
pyridine-4-carbaldehyde;
With
polymethylhydrosiloxane; iron(II) acetate; tricyclohexylphosphine;
In
tetrahydrofuran;
at 65 ℃;
for 16h;
With
sodium hydrogencarbonate;
In
tetrahydrofuran; methanol;
at 0 - 20 ℃;
Further stages.;
|
80% |
|
With
sodium tetrahydroborate;
In
methanol;
at 0 - 25 ℃;
for 2h;
|
80% |
|
With
Decaborane;
In
tetrahydrofuran; water;
at 20 ℃;
for 0.5h;
|
72% |
|
With
magnesium(II) perchlorate; 1-Benzyl-1,4-dihydronicotinamide;
In
acetonitrile;
at 80 ℃;
|
70% |
|
With
sodium tetrahydroborate;
In
methanol;
1) 0 deg C, 0.5 h, 2) reflux, 1 h;
|
68.7% |
|
With
trimethylamine-N-oxide; (1,4-dimethyl-5,7-diphenyl-1,2,3,4-tetrahydro-6H-cyclopenta[b]pyrazin-6-one) irontricarbonyl complex3; potassium formate;
In
ethanol;
at 45 - 60 ℃;
for 24h;
Inert atmosphere;
Schlenk technique;
|
66% |
|
With
formaldehyd; N,N,N',N'-tetramethylguanidine;
In
water;
at 20 ℃;
for 7h;
|
64% |
|
pyridine-4-carbaldehyde;
With
1-Methylpyrrolidine; 2-chloro-5-fluorophenylboronic acid; phenylsilane;
at 20 ℃;
for 16h;
Inert atmosphere;
With
sodium hydroxide;
In
water;
at 20 ℃;
for 2h;
chemoselective reaction;
|
63% |
|
With
diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate; tris[3,5-bis(trifluoromethyl)phenyl]-borane;
In
1,4-dioxane;
at 100 ℃;
for 12h;
Glovebox;
|
12% |
|
With
sodium tetrahydroborate;
for 0.0833333h;
|
|
|
With
isopropyl alcohol; potassium hydroxide;
at 85 ℃;
chemoselective reaction;
|
68 %Chromat. |
|
With
potassium hexachloropalatinate(IV); triethanolamine;
In
acetonitrile;
at 20 ℃;
for 24h;
Inert atmosphere;
Green light irradiation;
|
80 %Chromat. |
|
With
lead bismuth oxybromide; triethanolamine;
In
acetonitrile;
for 24h;
Inert atmosphere;
Irradiation;
|
54 %Chromat. |
|
With
glucose dehydrogenase; D-glucose; (R)-specific alcohol dehydrogenase from Candida maris IFO10003; NADH;
In
dimethyl sulfoxide;
at 30 ℃;
for 17h;
pH=6.5;
aq. phosphate buffer;
Enzymatic reaction;
|
|
|
With
sodium tetrahydroborate;
In
methanol;
at 0 - 20 ℃;
for 3h;
|
|
|
With
sodium tetrahydroborate;
In
methanol;
|
|
|
Multi-step reaction with 2 steps
1: C15H24Cl2N2Si / benzene / 1 h / 20 °C / Schlenk technique; Glovebox
2: silica gel / methanol / 60 °C
With
C15H24Cl2N2Si; silica gel;
In
methanol; benzene;
|
|
|
With
sodium tetrahydroborate;
In
methanol;
|
|
|
Multi-step reaction with 2 steps
1: two-dimensional iron(II) coordination polymer based on a divergent 4'-(4-diphenylamino)phenyl-4,2';6',4''-terpyridine ligand; potassium tert-butylate / neat (no solvent) / 0.5 h / 20 °C / Green chemistry
2: silica gel / ethyl acetate; hexane / 20 °C
With
two-dimensional iron(II) coordination polymer based on a divergent 4'-(4-diphenylamino)phenyl-4,2';6',4''-terpyridine ligand; potassium tert-butylate; silica gel;
In
hexane; ethyl acetate;
|
|
|
Multi-step reaction with 2 steps
1: Mn(2+)*C20H14N4*2Cl(1-); potassium tert-butylate / tetrahydrofuran / 2 h / 25 °C / Inert atmosphere; Glovebox
2: silica gel; water / ethyl acetate; hexane
With
potassium tert-butylate; Mn(2+)*C20H14N4*2Cl(1-); water; silica gel;
In
tetrahydrofuran; hexane; ethyl acetate;
|
|
|
Multi-step reaction with 2 steps
1: C30H46CoN4O2 / dichloromethane / 18 h / 40 °C / Inert atmosphere; Schlenk technique; Glovebox
2: tetrabutyl ammonium fluoride / dichloromethane; tetrahydrofuran / Inert atmosphere; Schlenk technique; Glovebox
With
tetrabutyl ammonium fluoride; C30H46CoN4O2;
In
tetrahydrofuran; dichloromethane;
|
586-95-8 Upstream products
-
3731-53-1
4-(aminomethyl)pyridine
-
55-22-1
pyridine-4-carboxylic acid
-
101990-69-6
(2,6-dichloropyridin-4-yl)methanol
-
1570-45-2

isonicotinic acid ethylester
586-95-8 Downstream products
-
1007-48-3
4-acetoxymethylpyridine
-
46721-93-1
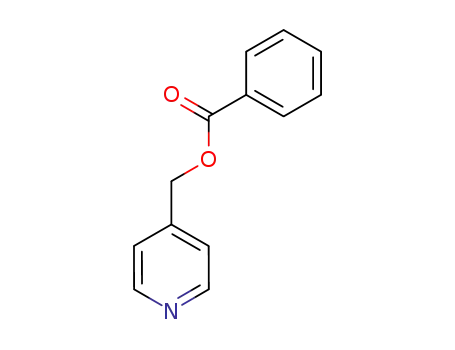
pyridin-4-ylmethyl benzoate
-
6457-74-5
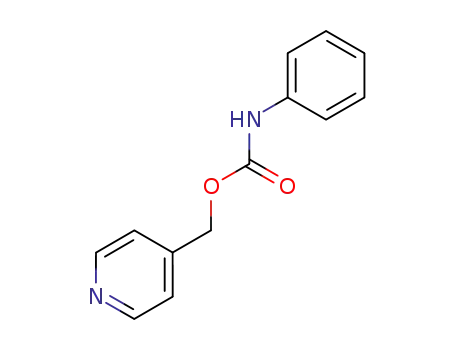
4-pyridylmethyl N-phenylcarbamate
-
6457-49-4
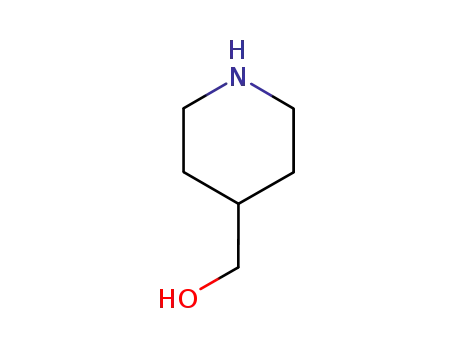
4-piperidinemethanol