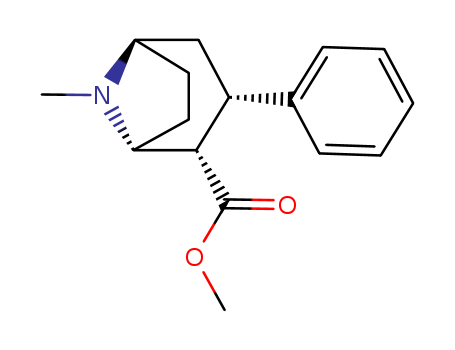
Troparil
Troparil
99% Purity Commercial production Troparil 74163-84-1 with Cheapest Price
- Molecular Formula:C16H21NO2
- Molecular Weight:259.348
- Vapor Pressure:4.47E-05mmHg at 25°C
- Refractive Index:1.54
- Boiling Point:350.2°C at 760 mmHg
- PKA:9.95±0.60(Predicted)
- Flash Point:121.3°C
- PSA:29.54000
- Density:1.099g/cm3
- LogP:2.36380
Troparil(Cas 74163-84-1) Usage
|
Pharmacokinetics |
Troparil is a few times more potent than cocaine as a dopamine reuptake inhibitor, but is less potent as a serotonin reuptake inhibitor, and has a duration spanning a few times longer, since the phenyl ring is directly connected to the tropane ring through a non-hydrolyzable carbon-carbon bond. The lack of an ester linkage removes the local anesthetic action from the drug, so troparil is a pure stimulant. This change in activity also makes troparil slightly less cardiotoxic than cocaine. |
InChI:InChI=1/C16H21NO2/c1-17-12-8-9-14(17)15(16(18)19-2)13(10-12)11-6-4-3-5-7-11/h3-7,12-15H,8-10H2,1-2H3/t12-,13+,14+,15-/m0/s1
74163-84-1 Relevant articles
Synthesis, radiosynthesis and first in vitro evaluation of novel PET-tracers for the dopamine transporter: [11C]IPCIT and [ 18F]FE@IPCIT
Rami-Mark, Christina,Bornatowicz, Birgit,Fink, Cornel,Otter, Paul,Ungersboeck, Johanna,Vraka, Chrysoula,Haeusler, Daniela,Nics, Lukas,Spreitzer, Helmut,Hacker, Marcus,Mitterhauser, Markus,Wadsak, Wolfgang
, p. 7562 - 7569 (2014/01/06)
Introduction Present data indicate that ...
Synthesis, ligand binding, QSAR, and CoMFA study of 3β-(p-substituted phenyl)tropane-2β-carboxylic acid methyl esters
Carroll,Gao,Rahman,Abraham,Parham,Lewin,Boja,Kuhar
, p. 2719 - 2725 (2007/10/02)
A series of 3β-(p-substituted phenyl)tro...
Compounds affecting the central nevous system.4. 3B-phenyltropane-2-carboxylic esters and analogs
Clarke et al.
, p. 1260,1262 (2007/10/04)
-
74163-84-1 Process route
-
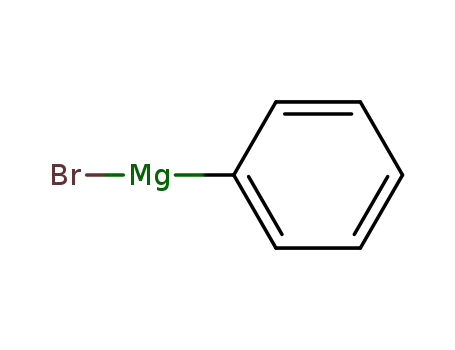
-
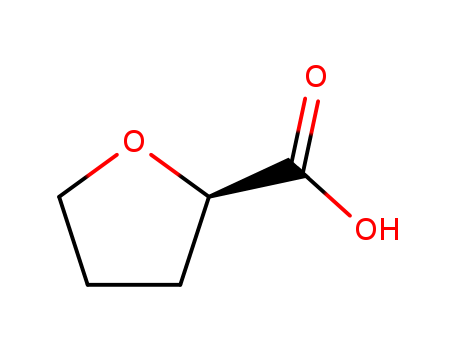
100-58-3
phenylmagnesium bromide

-
-
50373-10-9
methyl ecgonidine

-
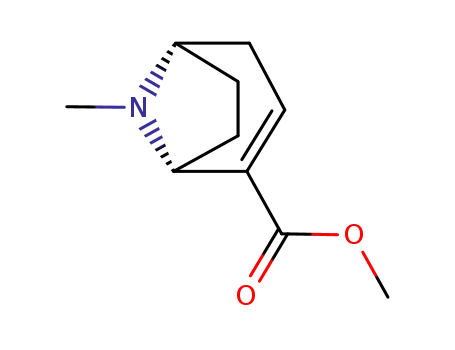
![(1R,3S,5S)-8-Methyl-3-phenyl-8-aza-bicyclo[3.2.1]octane-2-carboxylic acid methyl ester](/upload/2024/8/041947a4-b612-46a8-bb3e-ca6b4d95fb3a.png)
-
50370-54-2,50372-80-0,50583-05-6,57458-42-1,74163-83-0,74163-84-1,127379-25-3,127379-26-4
(1R,3S,5S)-8-Methyl-3-phenyl-8-aza-bicyclo[3.2.1]octane-2-carboxylic acid methyl ester
| Conditions | Yield |
|---|---|
|
phenylmagnesium bromide; methyl ecgonidine;
In
diethyl ether; dichloromethane;
at -78 - -40 ℃;
for 3.5h;
Inert atmosphere;
With
trifluoroacetic acid;
In
diethyl ether; dichloromethane;
Inert atmosphere;
|
65.28% |
-
-
cocaine HCl

-
![(1R,3S,5S)-8-Methyl-3-phenyl-8-aza-bicyclo[3.2.1]octane-2-carboxylic acid methyl ester](/upload/2024/8/041947a4-b612-46a8-bb3e-ca6b4d95fb3a.png)
-
50370-54-2,50372-80-0,50583-05-6,57458-42-1,74163-83-0,74163-84-1,127379-25-3,127379-26-4
(1R,3S,5S)-8-Methyl-3-phenyl-8-aza-bicyclo[3.2.1]octane-2-carboxylic acid methyl ester
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1.1: hydrogenchloride / 6 h / Reflux
1.2: 4 h / Reflux; Inert atmosphere
1.3: -40 - 20 °C
2.1: dichloromethane; diethyl ether / 3.5 h / -78 - -40 °C / Inert atmosphere
2.2: Inert atmosphere
With
hydrogenchloride;
In
diethyl ether; dichloromethane;
|
74163-84-1 Upstream products
-
100-58-3
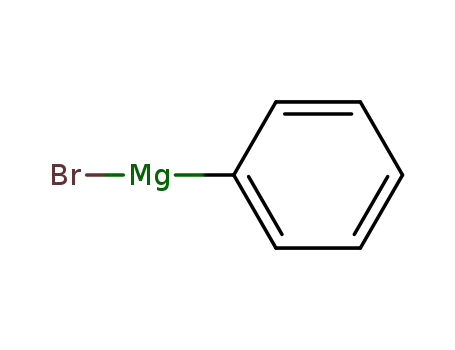
phenylmagnesium bromide
-
50373-10-9

methyl ecgonidine
-
50373-10-9
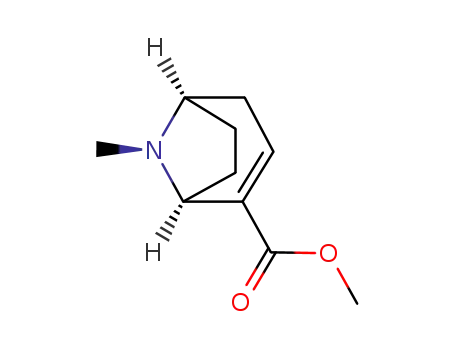
(R)-(-)-anhydroecgonine methyl ester
74163-84-1 Downstream products
-
127379-27-5
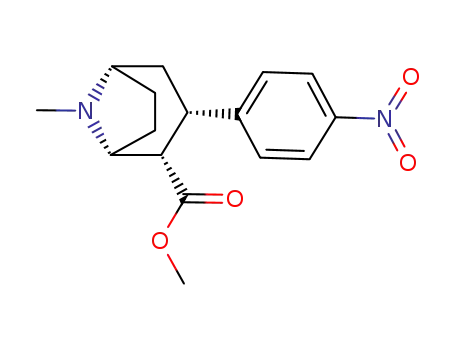
methyl (1R-2-exo-3-exo)-8-methyl-3-(4-nitrophenyl)-8-azabicyclo<3.2.1>octane-2-carboxylate
-
127279-72-5
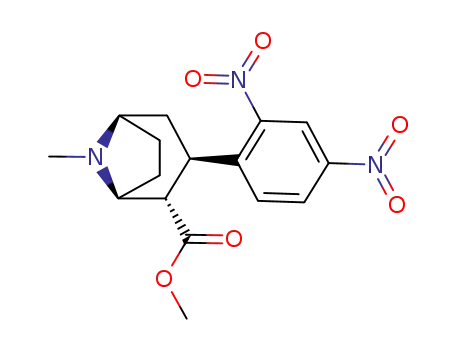
methyl (1RS-2-endo-3-exo)-3-(2,4-dinitrophenyl)-8-methyl-8-azabicyclo<3.2.1>octane-2-carboxylate
-
127279-73-6
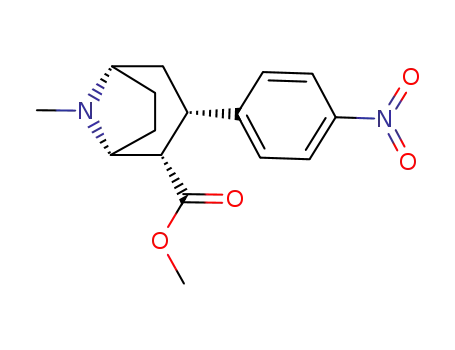
methyl (1RS-2-exo-3-exo)-8-methyl-3-(4-nitrophenyl)-8-azabicyclo<3.2.1>octane-2-carboxylate
-
135367-05-4
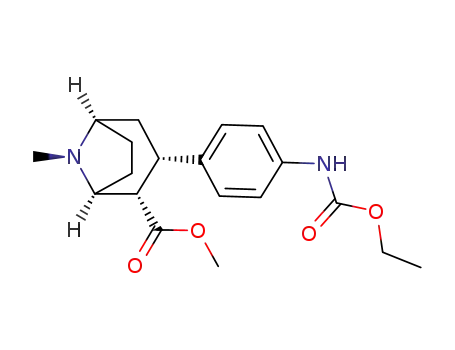
3β-(4-ethoxycarboxamidophenyl)tropane-2β-carboxylic acid methyl ester