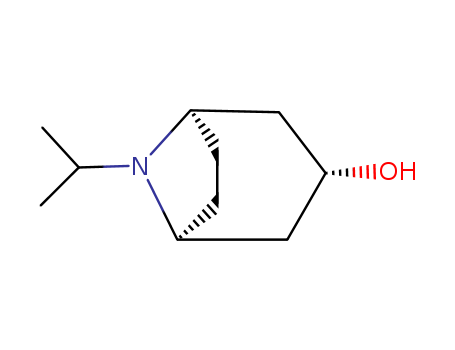
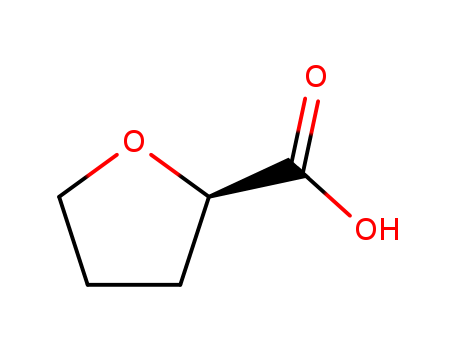
8-isopropyl-8-azabicyclo[3.2.1]octan-3-ol
8-isopropyl-8-azabicyclo[3.2.1]octan-3-ol
Quality Manufacturer Supply High Purity 99% 8-isopropyl-8-azabicyclo[3.2.1]octan-3-ol 3423-25-4 with Reasonable Price
- Molecular Formula:C10H19 N O
- Molecular Weight:169.267
- Vapor Pressure:0.00115mmHg at 25°C
- Boiling Point:266.9±15.0℃ (760 Torr)
- PKA:14.84±0.20(Predicted)
- Flash Point:89.7±14.5℃
- PSA:23.47000
- Density:1.037±0.06 g/cm3 (20 ºC 760 Torr)
- LogP:1.32040
endo-8-isopropyl-8-azabicyclo[3.2.1]octan-3-ol(Cas 3423-25-4) Usage
InChI:InChI=1/C10H19NO/c1-7(2)11-8-3-4-9(11)6-10(12)5-8/h7-10,12H,3-6H2,1-2H3
3423-25-4 Relevant articles
Metabolism of N-alkyldiamines and N-alkylnortropinones by transformed root cultures of Nicotiana and Brugmansia
Boswell, Henry D.,Draeger, Birgit,Eagles, John,McClintock, Carol,Parr, Adrian,Portsteffen, Andreas,Robins, David J.,Robins, Richard J.,Walton, Nicholas J.,Wong, Chi
, p. 855 - 869 (2007/10/03)
A range of analogues of N-methylputresci...
Specificities of the enzymes of N-alkyltropane biosynthesis in Brugmansia and Datura
Boswell, Henry D.,Draì?ger, Birgit,McLauchlan, W. Russell,Portsteffen, Andreas,Robins, David J.,Robins, Richard J.,Walton, Nicholas J.
, p. 871 - 878 (2007/10/03)
The enzymes N-methylputrescine oxidase (...
Conveniant Method for Replacement of Tertiary N-Methyl by Other Alkyl Groups: Application to Morphine Alkaloids
Manoharan, T. Samuel,Madyastha, K. Madhava,Singh, B. B.,Bhatnagar, S. P.,Weiss, Ulrich
, p. 5 - 11 (2007/10/02)
The replacement of N-methyl of N-methylp...
PHOSPHINERHODIUM COMPLEXES AS HOMOGENEOUS CATALYSTS. XVI. STEREOSELECTIVE HYDROGENATION OF CYCLIC KETONES
Toros, Szilard,Kollar, Laszlo,Heil, Balint,Marko, Laszlo
, p. 377 - 384 (2007/10/02)
Cyclic ketones have been hydrogenated st...
3423-25-4 Process route
-
![(1R,3R,5S)-3-Hydroxy-8-isopropyl-8-methyl-8-azonia-bicyclo[3.2.1]octane; iodide](/upload/2024/8/dc246809-518a-46b9-8fc5-d8533d45fab3.png)
-
93713-44-1
(1R,3R,5S)-3-Hydroxy-8-isopropyl-8-methyl-8-azonia-bicyclo[3.2.1]octane; iodide

-
-
3423-25-4
endo-8-(1-methylethyl)-8-azabicyclo<3.2.1>octan-3-ol

-
-
120-29-6
3-tropanol
| Conditions | Yield |
|---|---|
|
With
sodium thiophenolate;
In
acetonitrile; butanone;
Heating;
|
40% 13% |
-
![3-oxo-8-propyl-8-aza-bicyclo[3.2.1]octane-2,4-dicarboxylic acid](/upload/2024/8/1f4453b9-038c-4b10-9e96-2f5d36b23012.png)
-
3-oxo-8-propyl-8-aza-bicyclo[3.2.1]octane-2,4-dicarboxylic acid

-
-
3423-25-4
endo-8-(1-methylethyl)-8-azabicyclo<3.2.1>octan-3-ol
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: 6 M HCl / 1 h / 70 °C
2: Brugmansia candida x aurea / Enzymatic reaction
With
hydrogenchloride; Brugmansia candida x aurea;
1: Decarboxylation / 2: Reduction;
|
3423-25-4 Upstream products
-
3423-28-7
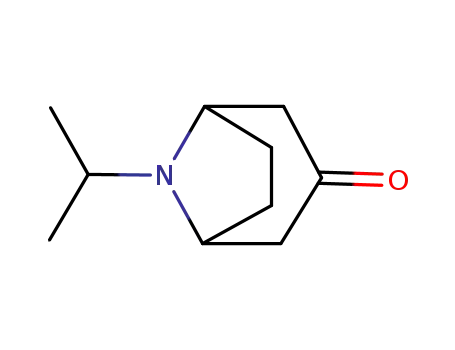
8-aza-8-isopropyl-bicyclo<3.2.1>octan-3-one
-
93713-44-1
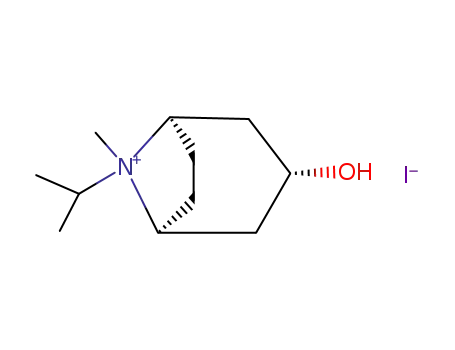
(1R,3R,5S)-3-Hydroxy-8-isopropyl-8-methyl-8-azonia-bicyclo[3.2.1]octane; iodide
3423-25-4 Downstream products
-
58005-18-8
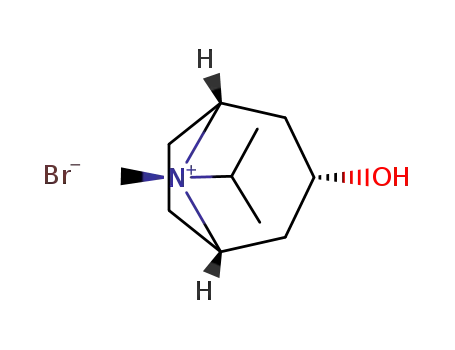
3-hydroxy-8-isopropyl-8-methyl-8-azonia-bicyclo[3.2.1]octane; bromide
-
145450-34-6
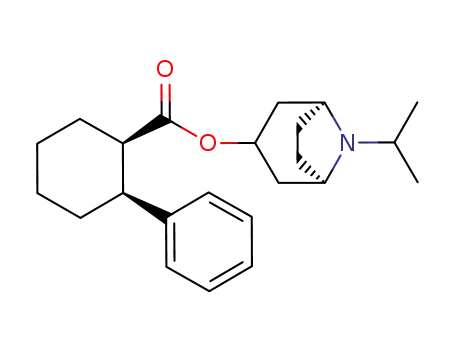
(1R,2S)-2-Phenyl-cyclohexanecarboxylic acid (1R,3R,5S)-8-isopropyl-8-aza-bicyclo[3.2.1]oct-3-yl ester
-
145450-03-9
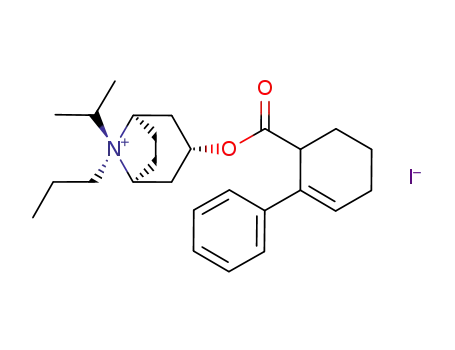
(1R,3R,5S,8R)-8-Isopropyl-3-(2-phenyl-cyclohex-2-enecarbonyloxy)-8-propyl-8-azonia-bicyclo[3.2.1]octane; iodide
-
145449-89-4
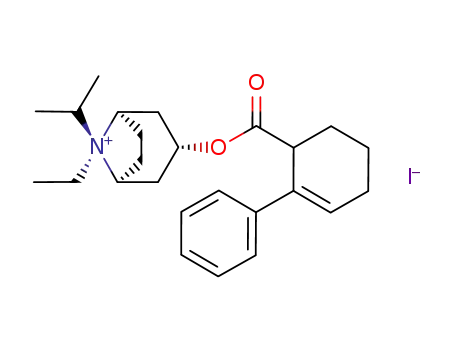
(1R,3R,5S,8R)-8-Ethyl-8-isopropyl-3-(2-phenyl-cyclohex-2-enecarbonyloxy)-8-azonia-bicyclo[3.2.1]octane; iodide