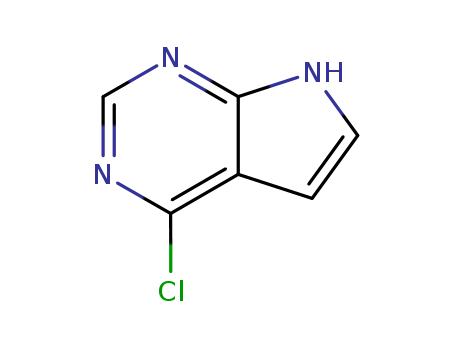
4-Chloropyrrolo[2,3-d]pyrimidine
4-Chloropyrrolo[2,3-d]pyrimidine
Factory Sells Best Quality 4-Chloropyrrolo[2,3-d]pyrimidine 3680-69-1 with USP
- Molecular Formula:C6H4ClN3
- Molecular Weight:153.571
- Appearance/Colour:light brown to brown crystalline powder
- Vapor Pressure:0Pa at 25℃
- Melting Point:183-184 °C
- Boiling Point:325.9 °C at 760 mmHg
- PKA:11.42±0.20(Predicted)
- Flash Point:180.7 °C
- PSA:41.57000
- Density:1.531 g/cm3
- LogP:1.61130
3680-69-1 Relevant articles
Synthesis and biological evaluation of some new tricyclic pyrrolo[3,2-e]tetrazolo[1,5-c]pyrimidine derivatives as potential antitubercular agents
Patil, Yogesh,Shingare, Ramesh,Choudhari, Amit,Borkute, Rachana,Sarkar, Dhiman,Madje, Balaji R.
, (2018)
A series of new tricyclic pyrrolo[3,2-e]...
Production process 4 -chloropyrrolo [2, 3 - d] pyrimidine
-
Paragraph 0108; 0113, (2021/10/27)
The production process of 4 - chloropyrr...
Production system of medical intermediate 4-chloropyrrolopyrimidine
-
Paragraph 0019; 0021-0025; 0029-0040, (2020/03/06)
The invention discloses a production sys...
Synthetic method of medical intermediate 4-chloropyrrolopyrimidine
-
Paragraph 0020; 0027-0028; 0030-0033; 0035, (2020/02/14)
The invention discloses a synthetic meth...
Method for preparing 4-chloro-7H-pyrrolo[2,3-d]pyrimidine by adopting non-phosphorus chlorination reagent
-
Paragraph 0062-0071, (2020/08/25)
The invention discloses a method for pre...
3680-69-1 Process route
-
-
3680-71-5
Allopurinol

-
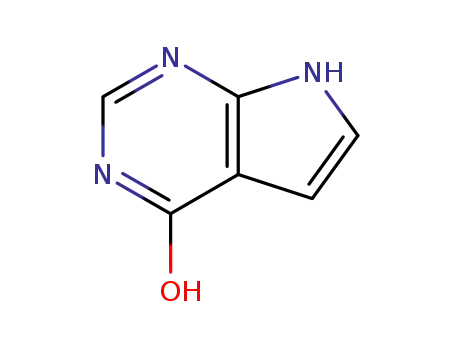
![4-chloro-1H-pyrrolo[2,3-d]pyrimidine](/upload/2024/8/d5575b4d-bb3d-44f6-8e59-42931848dde8.png)
-
3680-69-1
4-chloro-1H-pyrrolo[2,3-d]pyrimidine
| Conditions | Yield |
|---|---|
|
With
chlorine;
In
1-methyl-pyrrolidin-2-one; toluene;
at 160 - 180 ℃;
Temperature;
|
95% |
|
With
1,2,3-trichloropropane; chlorine;
In
1-methyl-pyrrolidin-2-one; toluene;
Reflux;
|
95% |
|
With
dmap; bis(trichloromethyl) carbonate;
In
chlorobenzene;
at 50 - 60 ℃;
for 6h;
Reagent/catalyst;
Solvent;
Temperature;
Inert atmosphere;
|
94.2% |
|
With
trichlorophosphate;
|
93% |
|
With
trichlorophosphate;
at 110 ℃;
for 1h;
|
91.5% |
|
With
oxalyl dichloride; N,N-dimethyl-formamide;
In
chlorobenzene;
at 60 - 70 ℃;
Reagent/catalyst;
Temperature;
Inert atmosphere;
|
90% |
|
With
trichlorophosphate;
at 85 ℃;
|
88% |
|
With
tetraethylammonium chloride; trichlorophosphate;
at 60 - 80 ℃;
for 0.5h;
|
87.6% |
|
With
trichlorophosphate;
In
toluene;
at 110 ℃;
Reagent/catalyst;
|
85% |
|
Allopurinol;
With
trichlorophosphate;
at 20 ℃;
Reflux;
With
potassium carbonate;
In
water;
at 20 ℃;
pH=7.5;
Cooling with ice;
|
67.8% |
|
With
trichlorophosphate;
at 100 ℃;
for 2.5h;
Inert atmosphere;
|
62% |
|
With
trichlorophosphate;
at 100 ℃;
for 2.5h;
|
62% |
|
Allopurinol;
With
trichlorophosphate;
for 1h;
Reflux;
In
dichloromethane; water;
for 24h;
|
52% |
|
Allopurinol;
With
trichlorophosphate;
for 1.5h;
Reflux;
With
water;
for 0.5h;
Cooling;
|
43.4% |
|
With
trichlorophosphate;
for 1.5h;
Heating / reflux;
|
42% |
|
With
trichlorophosphate;
for 1.5h;
Heating / reflux;
|
42% |
|
With
trichlorophosphate;
for 1.5h;
Heating / reflux;
|
42% |
|
With
trichlorophosphate;
for 1.5h;
Heating / reflux;
|
42% |
|
With
trichlorophosphate;
for 1.5h;
Heating / reflux;
|
42% |
|
With
trichlorophosphate;
Heating;
|
|
|
With
N-ethyl-N,N-diisopropylamine; trichlorophosphate;
In
toluene;
at 50 ℃;
|
82 g |
|
With
trichlorophosphate;
at 100 - 105 ℃;
for 1h;
|
10.2 g |
|
With
triethylamine; N-ethyl-N,N-diisopropylamine; trichlorophosphate;
at 50 ℃;
for 3h;
|
|
|
With
trichlorophosphate;
at 80 ℃;
for 4h;
|
-
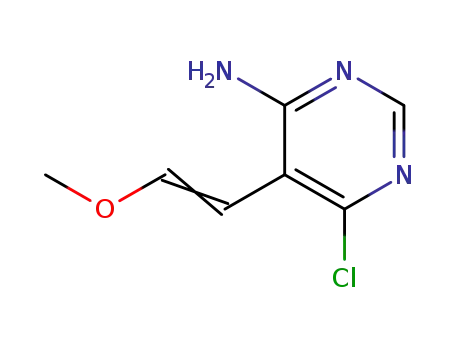
-
6-chloro-5-(2-methoxyvinyl)pyrimidin-4-ylamine

-
![4-chloro-1H-pyrrolo[2,3-d]pyrimidine](/upload/2024/8/d5575b4d-bb3d-44f6-8e59-42931848dde8.png)
-
3680-69-1
4-chloro-1H-pyrrolo[2,3-d]pyrimidine
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
tetrahydrofuran; water;
at 20 ℃;
for 7.5h;
Reflux;
|
94% |
|
6-chloro-5-(2-methoxyvinyl)pyrimidin-4-ylamine;
With
hydrogenchloride; water;
In
tetrahydrofuran;
at 20 ℃;
Reflux;
With
sodium hydrogencarbonate;
In
tetrahydrofuran; water;
at 20 ℃;
for 1h;
|
|
|
With
hydrogenchloride;
In
tetrahydrofuran; water;
for 7.5h;
Reflux;
|
54.5 g |
3680-69-1 Upstream products
-
3680-71-5
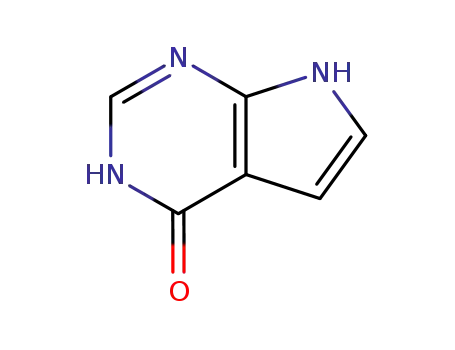
7-deazahypoxanthine
-
52133-67-2
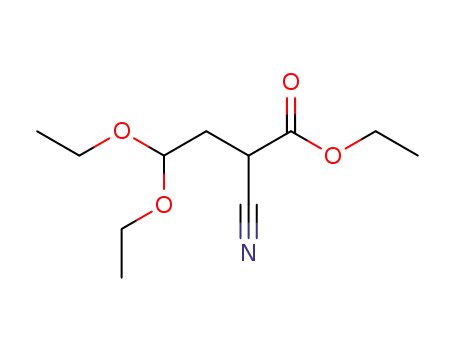
ethyl 2-cyano-4,4-diethoxybutyrate
-
7400-05-7
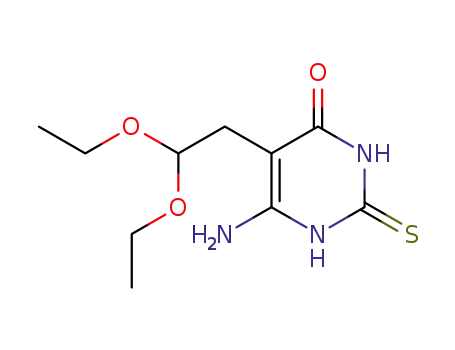
6-amino-5-(2,2-diethoxyethyl)-2-thioxo-2,3-dihydropyrimidin-4(1H)-one
-
67831-84-9
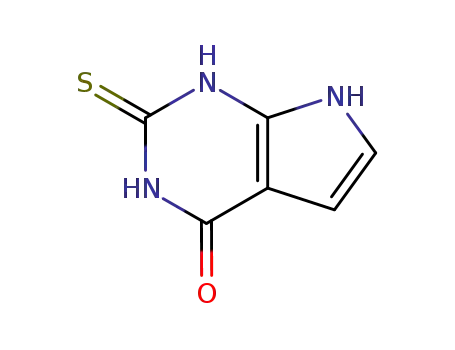
2-thioxo-1,2,3,7-tetrahydro-4H-pyrrolo[2,3-d]pyrimidin-4-one
3680-69-1 Downstream products
-
16019-34-4
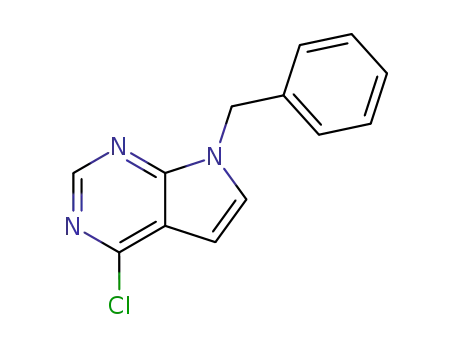
7-benzyl-4-chloro-7H-pyrrolo[2,3-d]pyrimidine
-
171620-43-2
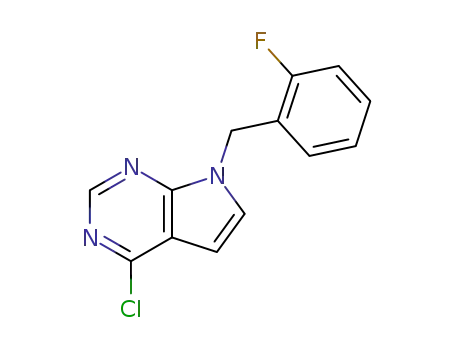
4-chloro-7-(2-fluorobenzyl)-7H-pyrrolo<2,3-d>pyrimidine
-
346600-33-7
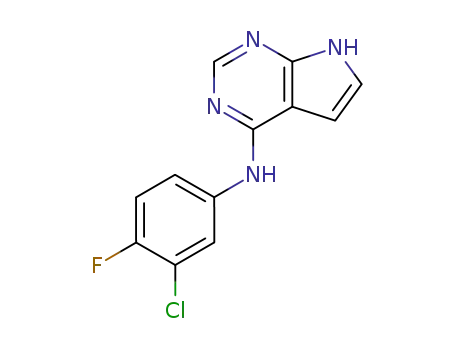
(3-chloro-4-fluoro-phenyl)-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-amine
-
865364-20-1
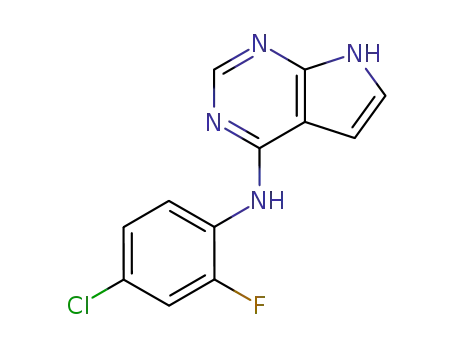
(4-chloro-2-fluoro-phenyl)-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-amine